In this issue
- Happy Holidays from ACE
- Over the past 12 months, ACE has delivered to your doorstep or inbox:
- New in 2017: JointHealth™ Education
- New research on biosimilars shows comparable safety and efficacy
- What is real world data?
- Assessing safety, efficacy of transitioning from originator biologic to biosimilar
- Influences on patient attitudes towards biosimmilars in Germany
- Have you been affected by new biosimilars policy?
JointHealth™ insight Published December 2016

This is one of our favorite times of the year in the Arthritis Consumer Experts office, filled with warmth and good cheer for each other, when we take the opportunity to reflect on our 17 years serving Canadians with arthritis – you, our members, subscribers and online visitors – and share our plans for 2017.
As Canada’s largest, longest running national patient-led arthritis organization and leading provider of evidence-based information and education programming, ACE thanks you for your continued interest, participation and engagement in our work in 2016. Through our JointHealth™ family of programs and the Arthritis Broadcast Network (ABN) we have made great progress to help the more than 5 million Canadians living with osteoarthritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, lupus, gout and the many other forms of the disease.
To continue to serve a broad arthritis community made up by so many different people – young, middle-aged and old – who have diverse needs that can change within a week or even a day. ACE will strive to provide the latest arthritis information and news, research and policy recommendations through our JointHealth™ and Arthritis Broadcast Network family of programs. Whether it’s supporting patients to deal with their healthcare professional, public formulary or private insurer, helping people reach out to their local elected official, or empowering them to maintain their work careers, ACE will work hard to help you in your journey with arthritis.
ACE is committed in 2017 to continue to work on behalf of Canadians living with arthritis to challenge and change the way people think about arthritis and ensure they fully understand our issues as a community. One of ACE’s approaches to this challenge will continue to be to dispel misunderstandings that make it difficult to affect positive change. For example, the still common misperception of arthritis pain as a “condition” associated with getting old and that it cannot be effectively treated, is holding patients back from setting higher expectations for improved health. These myths also hold policy makers back from doing more for our community. If they are not aware of the significant advances in diagnostic and treatment approaches that have occurred this past year, they lack motivation to increase spending on arthritis care and treatment.
People with arthritis need to voice their health and treatment concerns, and ACE will provide both leadership and support in doing so. We encourage you to engage with our communication channels and social media platform and for you to tell your stories in new ways to “reframe” arthritis issues from the ground up. The power to make change is with the grassroots. That is us; people living with arthritis.
Together, this holiday season we focus on caring for those on society’s margins: the sick and the hungry, the poor and the discriminated, the stranger in need of shelter. That spirit of caring also binds our arthritis community of patients and consumers together as we collectively confront the health and treatment concerns that face us. It is what the holidays are all about: Coming together as family to celebrate our accomplishments the past year and look forward to opportunities to build on our progress in the year ahead.
On behalf of my ACE team members and our Scientific, Medical and Consumer Advisory Board, I want to thank you again for your interest, participation and support of our work. We wish you a joyful holiday season and improved health in 2017.
Over the past 12 months,
ACE has delivered to your doorstep or inbox:
These accomplishments and more were imagined, developed and delivered by the ACE team, which is led by people living with arthritis and those who care for them.
New in 2017: JointHealth™ Education

In 2017, ACE will continue to build upon our existing, science-based information, education and support programs in both official languages and provide convenient access to available resources across Canada and social networks where patients can support, listen, and share personal stories with each other.
A key focus in 2017, in the context of the changing landscape of treatment options, will be to highlight the importance of the therapy conversation and the patient’s understanding of setting treatment goals.
JointHealth™ Education represents North America’s first ever program designed to formally educate through core curriculum and graduate modern arthritis patients with accredited status. In November 2016, ACE launched the first course - JointHealth™ Education: Rheumatoid Arthritis - to help patients with rheumatoid arthritis and their healthcare providers better tailor individual treatment plans and enable patients to meet their care goals.

In the first half of 2017, ACE will develop and launch two additional courses - JointHealth™ Education: PsA and JointHealth™ Education: AS - with a focus on educating and empowering people with psoriatic arthritis and ankylosing spondylitis by providing them with the most current, evidence-based information and communication training to facilitate productive two-way conversation between themselves and their rheumatologist (or other health care provider).
JointHealth™ Education is delivered through an easy-to-use, online “classroom” covering essential “lessons” to help people living with arthritis learn to have more meaningful, fact-based conversations with their rheumatologists, other health care team members, families, friends and employers. Because each of us learn at a different pace and through different educational tools, JointHealth™ Education helps people with RA learn from the comfort of their own home or over the work day lunch hour, through lessons, quizzes and “coaching” videos on their iPhone, iPad or computer.
Are you interested in going “back to school” to learn more about how to have full, satisfying conversations with your rheumatologist during your clinical visit? Click here, your class is about to begin: JointHealth™ Education.
 Biosimilars are becoming more commonly prescribed for autoimmune forms of arthritis and the body of data around their safety and efficacy continues to grow. Patient satisfaction has also been a frequent study topic. At the recent American College of Rheumatology Annual Meeting (ACR), ACE attended oral and poster presentations of studies that examine biosimilar use compared to their originator biologic.
Biosimilars are becoming more commonly prescribed for autoimmune forms of arthritis and the body of data around their safety and efficacy continues to grow. Patient satisfaction has also been a frequent study topic. At the recent American College of Rheumatology Annual Meeting (ACR), ACE attended oral and poster presentations of studies that examine biosimilar use compared to their originator biologic.
Norway's NOR-SWITCH study
Data from the first randomized trial of switching from an originator biologic to a biosimilar of the originator was presented at the ACR Annual Meeting by Norwegian researchers who led the NOR-SWITCH study1. The researchers found there were no differences in disease worsening between patients who were switched from the infliximab originator (Remicade) to the infliximab biosimilar Remsima (approved as Inflectra in Canada). Based on the researchers’ findings, disease worsening occurred in 26.2% of patients who stayed on infliximab (Remicade) and 29.6% of patients who switched to infliximab (Remsima).
Based on the researchers’ findings, disease worsening occurred in 26.2% of patients who stayed on infliximab (Remicade) and 29.6% of patients who switched to infliximab (Remsima).
Lead author Guro L. Goll, MD, a rheumatologist at Diakonhjemmet Hospital in Oslo, said: “I do think that the NOR-SWITCH study helps to build confidence in biosimilars as a concept, and I do think that our study supports that you can safely switch your infliximab originator Remicade patients to infliximab biosimilar Remsima even though we have not answered all questions, such as the multiple switching issue, and it would be nice to do further studies in gastroenterology patients as well.”
However, questions remain around the adoption of biosimilars, including:
In media interviews from the ACR annual meeting, Cheryl Koehn, President and Founder of Arthritis Consumer Experts, was one of several voices calling for the real need for real world data collection of the outcomes of transitioning back and forth between infliximab (Remicade) and infliximab (Inflectra), transitioning from one infliximab biosimilar to another infliximab biosimilar, and transitioning from other originator biologics to their biosimilars.
“In speaking to our Canadian rheumatology colleagues attending the ACR, we learned that there is growing confidence in the safety and efficacy of biosimilars, the newest class of biologics to come to market” said Koehn. “They, like ACE, are closely watching both the emerging clinical trial and real world data. Deciphering real world research results and understanding the impact biosimilars may – or may not – have on one, a few or thousands of patients is vitally important.”
And at the end of the day, scientists, rheumatologists and patients are looking for the same thing: Enough proof that a patient’s chance of responding to a biosimilar is high, and that their chance of the biosimilar losing its effectiveness or causing a rare but significant side effect is very low. The NOR-SWITCH trial offers some of that proof. But not all.
1Tore K. Kvien, Guro Lovik Goll, et. al., “Biosimilar Infliximab (CT-P13) Is Not Inferior to Originator Infliximab: Results From a 52-Week Randomized Switch Trial in Norway,” 2016 ACR/ARHP Annual Meeting. Abstract Number: 19L
Learn more about biosimilars research
To stay informed, go to these sources and use the search function for “biosimilars” for the latest research in North America and Europe:
American College of Rheumatology
http://www.rheumatology.org
The European League Against Rheumatism
http://www.eular.org
PubMed (National Center for Biotechnology Information)
http://www.ncbi.nlm.nih.gov/pubmed/
What is real world data?
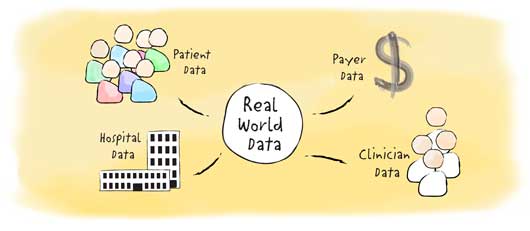 There are many, varied definitions of “real world data” (RWD). Essentially, RWD is a measurement of the efficacy of a medication after it receives approval by Health Canada, when prescribed and used in a practical, real-life settings that go beyond what is normally collected in pre-approval clinical trials and studies. RWD comes from various sources and includes patient data, data from clinicians, hospital data, data from payers and social data. Through its use alongside traditional data sources (such as clinical trials), RWD has the potential to provide new insights into medicines and their effects in the context of different larger patient populations.
There are many, varied definitions of “real world data” (RWD). Essentially, RWD is a measurement of the efficacy of a medication after it receives approval by Health Canada, when prescribed and used in a practical, real-life settings that go beyond what is normally collected in pre-approval clinical trials and studies. RWD comes from various sources and includes patient data, data from clinicians, hospital data, data from payers and social data. Through its use alongside traditional data sources (such as clinical trials), RWD has the potential to provide new insights into medicines and their effects in the context of different larger patient populations.
In the context of biosimilars, tracking the efficacy, safety and value to patients and the health care system of both originator biologics and their biosimilars is important. Patients and their physicians rely on this “real world data” when they are discussing and making treatment decisions.
Currently, there are a number of ongoing biosimilar studies, including “real world” tracking of patients, to monitor for any increase in immunogenicity when transitioning from an originator biologic to a biosimilar. This will help determine if transitioning patients can be considered a safe practice before it can be medically recommended or mandated through reimbursement policy.
When you are having a conversation about biosimilars as a treatment option with your healthcare professional or payer, ask them about these important real world data discussion points:
Assessing safety, efficacy of transitioning from originator biologic to biosimilar
A study summarizing the current literature presented at the 2016 ACR Annual Meeting has concluded transitioning from an originator biologic to a biosimilar for rheumatic diseases results in similar efficacy and safety data2.
With more biosimilars scheduled to enter the market in 2017 and beyond, clinical and real world data on the effects of transitioning are limited to transition studies of approved biosimilars. To address this gap in understanding the transitioning process – both originator to biosimilar and between biosimilars – Robert J. Moots, MB, BS, PhD, department of musculoskeletal biology, University of Liverpool and colleagues searched MEDLINE/Web of Science to identify studies where healthy volunteers or patients receiving infliximab, etanercept, adalimumab, or rituximab transitioned from originator biologics to their biosimilars.
In his conclusion, Dr. Moots said: “While initial transition data confirm maintenance of efficacy and safety, additional data from clinical and real world switching studies, especially switching between biosimilars, are required, as is continuing pharmacovigilance.” He added: “Any switching should remain a clinical decision made jointly by the treating physician and patient on an individual patient basis supported by scientific evidence.”
2 Robert Moots, et. al. “Switching to Biosimilars in Rheumatology Evidence-Based Practice,” 2016 ACR/AHRP Annual Meeting. Abstract Number: 639
Influences on patient attitudes towards biosimilars in Germany
Based on a survey of patients taking biosimilars and originator biologic patients in Germany (“Patient Attitudes Towards Being Prescribed Biosimilars in Inflammatory Autoimmune Diseases in Germany”), study authors found patients need a greater understanding of why they were prescribed biosimilars for rheumatoid arthritis, axial spondyloarthritis and psoriatic arthritis3.
Of 174 biosimilar patients, 78% were satisfied their disease was under control, compared to 85% of 87 patients receiving originator biologics. Biosimilar patients demonstrated lower understanding of their treatment with 39% stating they didn’t know enough about the medication when they started taking their treatment compared to 28% for patients receiving originator biologics. Patients lack of understanding of biosimilars was also found as 42% didn’t know their medication was based on an alternative originator product. Biosimilar patients who had not previously received an originator biologic (also referred to as biologic naïve patients) stated the most common reasons they accepted biosimilars was cost (30%) and physician’s recommendations (30%). Those patients who transitioned from an originator biologic to a biosimilar accepted the change due to their doctor’s recommendations (73%) and cost or insurance reasons (43%).
Commenting on the study’s real world finding of the lack of understanding surrounding patient attitudes to being prescribed biosimilars by their physicians, Cheryl Koehn said: “This study underlines the information gap and need for patient education on biosimilars. That is what led ACE to launch its Biosim•Exchange in September 2016. With research-based information and education, patients can have a full therapy conversation with their rheumatologist (or other arthritis specialist) to best decide on their choice of medication, including originator biologics and biosimilars.”

To view the Biosim•Exchange, please click here: http://biosim.jointhealth.org
3 James Piercy, et. al. “Patient Attitude Towards Being Prescribed Biosimilars in Inflammatory Autoimmune Diseases in Germany,” 2016 ACR/AHRP Annual Meeting. Abstract Number: 1428
Have you been affected by new biosimilars policy?
Provincial formularies and private health insurers have begun providing reimbursement for biosimilars approved in Canada. Have you had experience gaining reimbursement for a biosimilar prescription with a provincial or private health insurance formulary?
Get Involved

Biosimilars will have an increasing role to play in the Canadian healthcare system. How can you stay informed and involved?
Listening to you
We hope you find this information of use. Please tell us what you think by writing to us or emailing us at feedback@jointhealth.org. Through your ongoing and active participation, ACE can make its work more relevant to all Canadians living with arthritis.
Update your email or postal address
Please let us know of any changes by contacting ACE at feedback@jointhealth.org. This will ensure that you continue to receive your free email or print copy of JointHealth™ insight.
Arthritis Consumer Experts (ACE)
Who We Are
Arthritis Consumer Experts (ACE) provides research-based education, advocacy training, advocacy leadership and information to Canadians with arthritis. We help empower people living with all forms of arthritis to take control of their disease and to take action in healthcare and research decision making. ACE activities are guided by its members and led by people with arthritis, leading medical professionals and the ACE Advisory Board. To learn more about ACE, visit: www.jointhealth.org
Acknowledgements
Over the past 12 months, ACE received unrestricted grants-in-aid from: AbbVie Corporation, Amgen Canada, Arthritis Research Canada, AstraZeneca Canada, Canadian Institutes of Health Research, Celgene, Hoffman-La Roche Canada Ltd., Innovative Medicines Canada, Janssen Inc., Eli Lilly Canada, Merck Canada, Novartis, Pfizer Canada, Sanofi Canada, St. Paul’s Hospital (Vancouver), UCB Canada, and the University of British Columbia.
ACE also receives unsolicited donations from its community members (people with arthritis) across Canada.
ACE thanks funders for their support to help the nearly 5 million Canadians living with osteoarthritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and the many other forms of the disease. ACE assures its members, academic and healthcare professional collaborators, government and the public that the work of ACE is free from influence of its funders.
Disclaimer
The material contained on this website is provided for general information only. This website should not be relied on to suggest a course of treatment for a particular individual or as a substitute for consultation with qualified health professionals who are familiar with your individual medical needs. Should you have any healthcare related questions, you should contact your physician. You should never disregard medical advice or delay in seeking it because of something you have read on this or any website.
This site may provide links to other Internet sites only for the convenience of World Wide Web users. ACE is not responsible for the availability or content of these external sites, nor does ACE endorse, warrant or guarantee the products, services or information described or offered at these other Internet sites.
Although the information presented on this website is believed to be accurate at the time it is posted, this website could include inaccuracies, typographical errors or out-of-date information. This website may be changed at any time without prior notice.

A time to give thanks, reflect on the past and look forward to the future
This is one of our favorite times of the year in the Arthritis Consumer Experts office, filled with warmth and good cheer for each other, when we take the opportunity to reflect on our 17 years serving Canadians with arthritis – you, our members, subscribers and online visitors – and share our plans for 2017.
As Canada’s largest, longest running national patient-led arthritis organization and leading provider of evidence-based information and education programming, ACE thanks you for your continued interest, participation and engagement in our work in 2016. Through our JointHealth™ family of programs and the Arthritis Broadcast Network (ABN) we have made great progress to help the more than 5 million Canadians living with osteoarthritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, lupus, gout and the many other forms of the disease.
To continue to serve a broad arthritis community made up by so many different people – young, middle-aged and old – who have diverse needs that can change within a week or even a day. ACE will strive to provide the latest arthritis information and news, research and policy recommendations through our JointHealth™ and Arthritis Broadcast Network family of programs. Whether it’s supporting patients to deal with their healthcare professional, public formulary or private insurer, helping people reach out to their local elected official, or empowering them to maintain their work careers, ACE will work hard to help you in your journey with arthritis.
ACE is committed in 2017 to continue to work on behalf of Canadians living with arthritis to challenge and change the way people think about arthritis and ensure they fully understand our issues as a community. One of ACE’s approaches to this challenge will continue to be to dispel misunderstandings that make it difficult to affect positive change. For example, the still common misperception of arthritis pain as a “condition” associated with getting old and that it cannot be effectively treated, is holding patients back from setting higher expectations for improved health. These myths also hold policy makers back from doing more for our community. If they are not aware of the significant advances in diagnostic and treatment approaches that have occurred this past year, they lack motivation to increase spending on arthritis care and treatment.
People with arthritis need to voice their health and treatment concerns, and ACE will provide both leadership and support in doing so. We encourage you to engage with our communication channels and social media platform and for you to tell your stories in new ways to “reframe” arthritis issues from the ground up. The power to make change is with the grassroots. That is us; people living with arthritis.
Together, this holiday season we focus on caring for those on society’s margins: the sick and the hungry, the poor and the discriminated, the stranger in need of shelter. That spirit of caring also binds our arthritis community of patients and consumers together as we collectively confront the health and treatment concerns that face us. It is what the holidays are all about: Coming together as family to celebrate our accomplishments the past year and look forward to opportunities to build on our progress in the year ahead.
On behalf of my ACE team members and our Scientific, Medical and Consumer Advisory Board, I want to thank you again for your interest, participation and support of our work. We wish you a joyful holiday season and improved health in 2017.
 |
Sincerely, Cheryl Koehn Person with rheumatoid arthritis Founder and President of Arthritis Consumer Experts |
Over the past 12 months,
ACE has delivered to your doorstep or inbox:
| JointHealth™ Education - Rheumatoid Arthritis |
| Biosim•Exchange |
| Walk10Blocks research app |
| 10 JointHealth™ insights |
| 88 JointHealth™ expresses |
| 124 ABN articles |
| 203 ACE and ABN posts |
| 1296 ACE, ABN, Cheryl Koehn Tweets |
| Multiple National and International Conference Attendance and Presentations |
New in 2017: JointHealth™ Education

In 2017, ACE will continue to build upon our existing, science-based information, education and support programs in both official languages and provide convenient access to available resources across Canada and social networks where patients can support, listen, and share personal stories with each other.
A key focus in 2017, in the context of the changing landscape of treatment options, will be to highlight the importance of the therapy conversation and the patient’s understanding of setting treatment goals.
JointHealth™ Education represents North America’s first ever program designed to formally educate through core curriculum and graduate modern arthritis patients with accredited status. In November 2016, ACE launched the first course - JointHealth™ Education: Rheumatoid Arthritis - to help patients with rheumatoid arthritis and their healthcare providers better tailor individual treatment plans and enable patients to meet their care goals.

In the first half of 2017, ACE will develop and launch two additional courses - JointHealth™ Education: PsA and JointHealth™ Education: AS - with a focus on educating and empowering people with psoriatic arthritis and ankylosing spondylitis by providing them with the most current, evidence-based information and communication training to facilitate productive two-way conversation between themselves and their rheumatologist (or other health care provider).
JointHealth™ Education is delivered through an easy-to-use, online “classroom” covering essential “lessons” to help people living with arthritis learn to have more meaningful, fact-based conversations with their rheumatologists, other health care team members, families, friends and employers. Because each of us learn at a different pace and through different educational tools, JointHealth™ Education helps people with RA learn from the comfort of their own home or over the work day lunch hour, through lessons, quizzes and “coaching” videos on their iPhone, iPad or computer.
Are you interested in going “back to school” to learn more about how to have full, satisfying conversations with your rheumatologist during your clinical visit? Click here, your class is about to begin: JointHealth™ Education.
 Biosimilars are becoming more commonly prescribed for autoimmune forms of arthritis and the body of data around their safety and efficacy continues to grow. Patient satisfaction has also been a frequent study topic. At the recent American College of Rheumatology Annual Meeting (ACR), ACE attended oral and poster presentations of studies that examine biosimilar use compared to their originator biologic.
Biosimilars are becoming more commonly prescribed for autoimmune forms of arthritis and the body of data around their safety and efficacy continues to grow. Patient satisfaction has also been a frequent study topic. At the recent American College of Rheumatology Annual Meeting (ACR), ACE attended oral and poster presentations of studies that examine biosimilar use compared to their originator biologic.Norway's NOR-SWITCH study
Data from the first randomized trial of switching from an originator biologic to a biosimilar of the originator was presented at the ACR Annual Meeting by Norwegian researchers who led the NOR-SWITCH study1. The researchers found there were no differences in disease worsening between patients who were switched from the infliximab originator (Remicade) to the infliximab biosimilar Remsima (approved as Inflectra in Canada).
 Based on the researchers’ findings, disease worsening occurred in 26.2% of patients who stayed on infliximab (Remicade) and 29.6% of patients who switched to infliximab (Remsima).
Based on the researchers’ findings, disease worsening occurred in 26.2% of patients who stayed on infliximab (Remicade) and 29.6% of patients who switched to infliximab (Remsima).Lead author Guro L. Goll, MD, a rheumatologist at Diakonhjemmet Hospital in Oslo, said: “I do think that the NOR-SWITCH study helps to build confidence in biosimilars as a concept, and I do think that our study supports that you can safely switch your infliximab originator Remicade patients to infliximab biosimilar Remsima even though we have not answered all questions, such as the multiple switching issue, and it would be nice to do further studies in gastroenterology patients as well.”
However, questions remain around the adoption of biosimilars, including:
- Will biosimilars rates of infusion and/or injection site reactions be similar over the long-term?
- Will biosimilars rates and types of serious infections parallel their originators?
- What happens to people’s immune systems when they make multiple transitions from originator biologic to its biosimilar, and then to another originator biologic or biosimilar with a different mechanism of action?
In media interviews from the ACR annual meeting, Cheryl Koehn, President and Founder of Arthritis Consumer Experts, was one of several voices calling for the real need for real world data collection of the outcomes of transitioning back and forth between infliximab (Remicade) and infliximab (Inflectra), transitioning from one infliximab biosimilar to another infliximab biosimilar, and transitioning from other originator biologics to their biosimilars.
“In speaking to our Canadian rheumatology colleagues attending the ACR, we learned that there is growing confidence in the safety and efficacy of biosimilars, the newest class of biologics to come to market” said Koehn. “They, like ACE, are closely watching both the emerging clinical trial and real world data. Deciphering real world research results and understanding the impact biosimilars may – or may not – have on one, a few or thousands of patients is vitally important.”
And at the end of the day, scientists, rheumatologists and patients are looking for the same thing: Enough proof that a patient’s chance of responding to a biosimilar is high, and that their chance of the biosimilar losing its effectiveness or causing a rare but significant side effect is very low. The NOR-SWITCH trial offers some of that proof. But not all.
1Tore K. Kvien, Guro Lovik Goll, et. al., “Biosimilar Infliximab (CT-P13) Is Not Inferior to Originator Infliximab: Results From a 52-Week Randomized Switch Trial in Norway,” 2016 ACR/ARHP Annual Meeting. Abstract Number: 19L
Learn more about biosimilars research
To stay informed, go to these sources and use the search function for “biosimilars” for the latest research in North America and Europe:
American College of Rheumatology
http://www.rheumatology.org
The European League Against Rheumatism
http://www.eular.org
PubMed (National Center for Biotechnology Information)
http://www.ncbi.nlm.nih.gov/pubmed/
What is real world data?
 There are many, varied definitions of “real world data” (RWD). Essentially, RWD is a measurement of the efficacy of a medication after it receives approval by Health Canada, when prescribed and used in a practical, real-life settings that go beyond what is normally collected in pre-approval clinical trials and studies. RWD comes from various sources and includes patient data, data from clinicians, hospital data, data from payers and social data. Through its use alongside traditional data sources (such as clinical trials), RWD has the potential to provide new insights into medicines and their effects in the context of different larger patient populations.
There are many, varied definitions of “real world data” (RWD). Essentially, RWD is a measurement of the efficacy of a medication after it receives approval by Health Canada, when prescribed and used in a practical, real-life settings that go beyond what is normally collected in pre-approval clinical trials and studies. RWD comes from various sources and includes patient data, data from clinicians, hospital data, data from payers and social data. Through its use alongside traditional data sources (such as clinical trials), RWD has the potential to provide new insights into medicines and their effects in the context of different larger patient populations.In the context of biosimilars, tracking the efficacy, safety and value to patients and the health care system of both originator biologics and their biosimilars is important. Patients and their physicians rely on this “real world data” when they are discussing and making treatment decisions.
Currently, there are a number of ongoing biosimilar studies, including “real world” tracking of patients, to monitor for any increase in immunogenicity when transitioning from an originator biologic to a biosimilar. This will help determine if transitioning patients can be considered a safe practice before it can be medically recommended or mandated through reimbursement policy.
When you are having a conversation about biosimilars as a treatment option with your healthcare professional or payer, ask them about these important real world data discussion points:
- What has been your experience with prescribing biosimilars?
- Are you satisfied they are a safe and effective treatment option for me? Why?
- Does the biosimilar you are recommending to me have a patient support program?
- Where can I find reliable, patient-friendly reading material on biosimilars?
Assessing safety, efficacy of transitioning from originator biologic to biosimilar
A study summarizing the current literature presented at the 2016 ACR Annual Meeting has concluded transitioning from an originator biologic to a biosimilar for rheumatic diseases results in similar efficacy and safety data2.
With more biosimilars scheduled to enter the market in 2017 and beyond, clinical and real world data on the effects of transitioning are limited to transition studies of approved biosimilars. To address this gap in understanding the transitioning process – both originator to biosimilar and between biosimilars – Robert J. Moots, MB, BS, PhD, department of musculoskeletal biology, University of Liverpool and colleagues searched MEDLINE/Web of Science to identify studies where healthy volunteers or patients receiving infliximab, etanercept, adalimumab, or rituximab transitioned from originator biologics to their biosimilars.
In his conclusion, Dr. Moots said: “While initial transition data confirm maintenance of efficacy and safety, additional data from clinical and real world switching studies, especially switching between biosimilars, are required, as is continuing pharmacovigilance.” He added: “Any switching should remain a clinical decision made jointly by the treating physician and patient on an individual patient basis supported by scientific evidence.”
2 Robert Moots, et. al. “Switching to Biosimilars in Rheumatology Evidence-Based Practice,” 2016 ACR/AHRP Annual Meeting. Abstract Number: 639
Influences on patient attitudes towards biosimilars in Germany
Based on a survey of patients taking biosimilars and originator biologic patients in Germany (“Patient Attitudes Towards Being Prescribed Biosimilars in Inflammatory Autoimmune Diseases in Germany”), study authors found patients need a greater understanding of why they were prescribed biosimilars for rheumatoid arthritis, axial spondyloarthritis and psoriatic arthritis3.
Of 174 biosimilar patients, 78% were satisfied their disease was under control, compared to 85% of 87 patients receiving originator biologics. Biosimilar patients demonstrated lower understanding of their treatment with 39% stating they didn’t know enough about the medication when they started taking their treatment compared to 28% for patients receiving originator biologics. Patients lack of understanding of biosimilars was also found as 42% didn’t know their medication was based on an alternative originator product. Biosimilar patients who had not previously received an originator biologic (also referred to as biologic naïve patients) stated the most common reasons they accepted biosimilars was cost (30%) and physician’s recommendations (30%). Those patients who transitioned from an originator biologic to a biosimilar accepted the change due to their doctor’s recommendations (73%) and cost or insurance reasons (43%).
Commenting on the study’s real world finding of the lack of understanding surrounding patient attitudes to being prescribed biosimilars by their physicians, Cheryl Koehn said: “This study underlines the information gap and need for patient education on biosimilars. That is what led ACE to launch its Biosim•Exchange in September 2016. With research-based information and education, patients can have a full therapy conversation with their rheumatologist (or other arthritis specialist) to best decide on their choice of medication, including originator biologics and biosimilars.”

To view the Biosim•Exchange, please click here: http://biosim.jointhealth.org
3 James Piercy, et. al. “Patient Attitude Towards Being Prescribed Biosimilars in Inflammatory Autoimmune Diseases in Germany,” 2016 ACR/AHRP Annual Meeting. Abstract Number: 1428
Have you been affected by new biosimilars policy?
Provincial formularies and private health insurers have begun providing reimbursement for biosimilars approved in Canada. Have you had experience gaining reimbursement for a biosimilar prescription with a provincial or private health insurance formulary?
Get Involved
Biosimilars will have an increasing role to play in the Canadian healthcare system. How can you stay informed and involved?
- Provide your comments and questions to Arthritis Consumer Experts at feedback@jointhealth.org
- Continue to share perspectives and experiences around this important arthritis treatment topic
- Help educate your community – they will be affected by the arrival of biosimilars
- Understand how biosimilars are assessed and listed on public and private formularies
Listening to you
We hope you find this information of use. Please tell us what you think by writing to us or emailing us at feedback@jointhealth.org. Through your ongoing and active participation, ACE can make its work more relevant to all Canadians living with arthritis.
Update your email or postal address
Please let us know of any changes by contacting ACE at feedback@jointhealth.org. This will ensure that you continue to receive your free email or print copy of JointHealth™ insight.
Arthritis Consumer Experts (ACE)
Who We Are
Arthritis Consumer Experts (ACE) provides research-based education, advocacy training, advocacy leadership and information to Canadians with arthritis. We help empower people living with all forms of arthritis to take control of their disease and to take action in healthcare and research decision making. ACE activities are guided by its members and led by people with arthritis, leading medical professionals and the ACE Advisory Board. To learn more about ACE, visit: www.jointhealth.org
Acknowledgements
Over the past 12 months, ACE received unrestricted grants-in-aid from: AbbVie Corporation, Amgen Canada, Arthritis Research Canada, AstraZeneca Canada, Canadian Institutes of Health Research, Celgene, Hoffman-La Roche Canada Ltd., Innovative Medicines Canada, Janssen Inc., Eli Lilly Canada, Merck Canada, Novartis, Pfizer Canada, Sanofi Canada, St. Paul’s Hospital (Vancouver), UCB Canada, and the University of British Columbia.
ACE also receives unsolicited donations from its community members (people with arthritis) across Canada.
ACE thanks funders for their support to help the nearly 5 million Canadians living with osteoarthritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and the many other forms of the disease. ACE assures its members, academic and healthcare professional collaborators, government and the public that the work of ACE is free from influence of its funders.
Disclaimer
The material contained on this website is provided for general information only. This website should not be relied on to suggest a course of treatment for a particular individual or as a substitute for consultation with qualified health professionals who are familiar with your individual medical needs. Should you have any healthcare related questions, you should contact your physician. You should never disregard medical advice or delay in seeking it because of something you have read on this or any website.
This site may provide links to other Internet sites only for the convenience of World Wide Web users. ACE is not responsible for the availability or content of these external sites, nor does ACE endorse, warrant or guarantee the products, services or information described or offered at these other Internet sites.
Although the information presented on this website is believed to be accurate at the time it is posted, this website could include inaccuracies, typographical errors or out-of-date information. This website may be changed at any time without prior notice.
