In this issue
- Arthritis Consumer Experts is pleased to publish its ninth annual JointHealth™ Arthritis Medications Report Card
- What can patients like you do?
- New arthritis medications on the horizon
- National Pharmacare:
Where do patients fit in the conversation? - National Pharmacare:
Time for patients to get involved - What do ACE members, subscribers and followers want?
- For public and private payers, ACE's key considerations towards the framework of a national pharmacare program include:

Arthritis Consumer Experts is pleased to publish its ninth annual JointHealth™ Arthritis Medications Report Card
 Every year, our Report Card helps Canadians and their rheumatologists identify how their province measures up to other provinces in terms of providing reimbursement access to medications approved for autoimmune forms of arthritis.
Every year, our Report Card helps Canadians and their rheumatologists identify how their province measures up to other provinces in terms of providing reimbursement access to medications approved for autoimmune forms of arthritis. The Report Card empowers consumers (patients) to become active participants in their own disease treatment plan. It provides important information that consumers can use to discuss with their rheumatologists, which Health Canada approved medication(s) might be best suited for their disease, health history and literacy, physical limitations, cultural beliefs and ability to administer or take the medication(s).
The current Report Card ranks twelve publicly funded medication formularies based on the number of medically necessary arthritis medications they list out of a possible 13 medication treatments. The medications that ACE monitors fall into three categories: biologic response modifiers ("biologics"), subsequent entry biologics ("SEBs"), and targeted small molecule medications ("TSMMs").
The current and historical ranking of each province is shown on the Report Card. As each makes decisions, the on-line version of the Report Card is updated, giving ACE members, subscribers and followers the latest information available.
What can patients like you do?
Arthritis Consumer Experts encourages you to share the findings in the Report Card with your healthcare team and federal and provincial elected representative. Copies of the JointHealth™ Arthritis Medications Report Card can be found in every rheumatologist's office in Canada. You may also email feedback@jointhealth.org to request a copy of the Report Card. ACE will update its members, subscribers and stakeholders on changes as they occur in reimbursement access through our online Report Card.
If you feel your province doesn't measure up, write and speak to your elected provincial or federal representative about the lack of equitable reimbursement access and patient/physician choice in treating autoimmune forms of arthritis. For tips on how to communicate with elected officials or key decision-makers, please visit our "What You Can Do" page within the "Taking Action" section of the JointHealth™ website.

More than 4.6 million Canadians live with arthritis; by 2036, an estimated 7.5 million Canadian adults will have arthritis. Arthritis is the leading cause of disability and work disability in Canada. The impact of arthritis on the Canadian economy in healthcare costs and lost productivity is estimated to be $33 billion each year. However, no comprehensive model of arthritis care is available. Medication coverage across the different provinces varies. The Report Card highlights to government decision-makers this lack of equitable access and patient/physician choice in treating the different types of autoimmune arthritis.
As medication treatment advanced, so did the individual provincial medication formularies. Our 2007 Report Card ranked just six biologic response modifiers available at the time. In 2010, we identified three new biologics approved for sale and use in Canada, and added juvenile idiopathic arthritis in the disease category. In 2014, we began tracking two new classes of medications - subsequent entry biologics and targeted small molecule medications.
| Medication Treatment | US Status | Canadian Status |
| baricitinib is an oral JAK1 and JAK2 inhibitor |
|
|
| apremilast (Otezla) is an oral, small-molecule compound, taken as two tablets twice a day. |
|
|
| secukinumab (Cosentyx) is a human monoclonal antibody manufactured by Novartis Pharma AG. |
|
|
Thanks to the advocacy efforts of people living with arthritis and their rheumatologists, progress has been made in 2015 in many Canadian provinces. Patients and caregivers are valuable in the medication review process when they participate in national and regional patient input review. These reviews help healthcare policy makers understand how effective a medication is.
Looking at medication treatments that are available in different countries can provide an idea of emerging arthritis medications in Canada. Furthermore, medications that are currently approved for one form of arthritis may gain approval for use to treat another form of arthritis. ACE provides this short summary of medications that are in the pipeline for people in Canada.
National Pharmacare:
Where do patients fit in the conversation?
Canadian spending on medications continues to top that of most industrialized countries, according to a new report on international health trends. The study from the Organization for Economic Co-operation and Development (OECD) ranks Canada as the fourth-highest spender on pharmaceutical medications among 29 countries when measured by population. Pharmaceutical medication spending in Canada worked out to $713 (U.S.) a person in 2013, well above the OECD average of $515. The highest spender by far was the United States at $1,026, followed by Japan and Greece. Drug costs were lowest in Denmark at $240 a person.
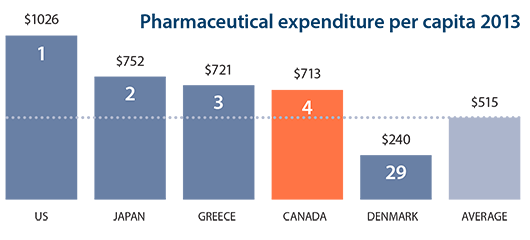
The "Health at a Glance" study is released every two years and provides a report card on a range of measures that compare population health and the performance of health systems in OECD countries. Through a combination of measures, it attempts to rank access and quality of care, as well as health status and healthcare resources.
The OECD report card found Canada has one of the lowest percentages of public drug plan coverage for pharmaceutical costs. The current structure of prescription drug coverage for Canadians is a patchwork of drug programs, including public and private insurance, as well as direct payment by individuals. The OECD report card has come out as pressure is mounting to expand public drug plan coverage of prescription drugs in Canada.
In Canada, as many as one in 10 seniors skips buying medications because they can't afford them, according to a Conference Board of Canada study released this fall, sponsored by the Canadian Medical Association. The CMA has called for catastrophic coverage for prescription drugs that would kick in for those households that spend more than $1,500 a year, or 3 per cent of their income, on prescription medications.
Through the years, the JointHealth™ Arthritis Medications Report Card has illustrated that who is covered by public drug plans in Canada varies from province to province. This framework has widespread inconsistencies and inequities, often resulting in compromised patient care and outcomes at great cost to individuals, the healthcare systems, and society as a whole.
National Pharmacare:
Time for patients to get involved
Canada is one of the few industrialized countries with universal health insurance that does not offer a comprehensive program for medically necessary drugs.
Over the past 18 months, elected officials, experts, health leaders and patient groups have increasingly begun sharing their knowledge and insights to discussion about pharmacare and publicly calling for a pan-Canadian program.
|
"A national pharmacare program is in the interest of our patients and of all Canadians." - Dr. Eric Hoskins |
In June 2015, a Roundtable Discussion on a pan-Canadian pharmacare program was led by Dr. Eric Hoskins, Ontario's Minister of Health and Long-Term Care, and attended by the Ministers of Health from the Northwest Territories, British Columbia, Saskatchewan, Manitoba, New Brunswick, and Newfoundland and Labrador.
| Steve Kent, the Health Minister for Newfoundland and Labrador, claims the federal government has "a critical role to play. This is not something, quite frankly, that provinces and territories can do on their own." |
Although the Green Party and the NDP made it part of their platforms in the recent federal election, the incoming Liberal government pledged only to improve access to prescription medications, and to work with provincial and territorial governments to buy drugs in bulk as a way to make them more affordable. However, in Prime Minister Trudeau's Mandate Letter to the Minister of Health, the Prime Minister expects the Minister to "explore the need for a national formulary".
What do ACE members, subscribers and followers want?
In a joint statement issued after the Roundtable, the Ministers of Health commented: "This is neither the beginning nor the end of this discussion. To better inform ourselves with the evidence, we are determined to seek input from other voices who can offer guidance and advice as we move this conversation forward." ACE supports any initiative to improve access to health care and financial assistance for medications, and advocates that all governments and policy makers seek advice and guidance from patients.
As the national pharmacare conversation continues at both the provincial and federal levels, ACE encourages arthritis consumers to get involved and tell their elected officials what patient needs must be met in a national pharmacare program. ACE will also be meeting with private insurers and large employers who provide reimbursement for the private costs of medicines and will also be part of any future dialogues about national pharmacare.
- Consistency and universality of coverage: Patients have a right to reimbursement access to medically necessary medications.
- Protection of therapeutic: Patients have a right to choose with their specialist among a full range of research-based medication treatments, and be assured of a secure supply of the full compliment of Health Canada approved medications, including both long-standing compounds and more recently approved therapy advancements.
- Timely access: Reimbursement decisions, whether by public or private payer, must not exceed best evidence and clinical guideline timelines to ensure that the opportunity to treat most effectively is not lost.
Listening to you
We hope you find this information of use. Please tell us what you think by writing to us or emailing us at info@jointhealth.org. Through your ongoing and active participation, ACE can make its work more relevant to all Canadians living with arthritis.
Update your email or postal address
Please let us know of any changes by contacting ACE at info@jointhealth.org. This will ensure that you continue to receive your free email or print copy of JointHealth™ monthly.
Arthritis Consumer Experts (ACE)
Who We Are
Arthritis Consumer Experts (ACE) provides research-based education, advocacy training, advocacy leadership and information to Canadians with arthritis. We help empower people living with all forms of arthritis to take control of their disease and to take action in healthcare and research decision making. ACE activities are guided by its members and led by people with arthritis, leading medical professionals and the ACE Advisory Board. To learn more about ACE, visit: www.jointhealth.org
Acknowledgements
Over the past 12 months, ACE received unrestricted grants-in-aid from: AbbVie Corporation, Amgen Canada, Arthritis Research Canada, Canadian Institutes of Health Research, Celgene Inc., Hoffman-La Roche Canada Ltd., Janssen Inc., Merck & Co., Inc., Pfizer Canada, Sanofi Canada, UCB Canada Inc. and the University of British Columbia. ACE also receives unsolicited donations from its community members (people with arthritis) across Canada.
ACE thanks these private and public organizations and individuals.
Disclaimer
The material contained on this website is provided for general information only. This website should not be relied on to suggest a course of treatment for a particular individual or as a substitute for consultation with qualified health professionals who are familiar with your individual medical needs. Should you have any healthcare related questions, you should contact your physician. You should never disregard medical advice or delay in seeking it because of something you have read on this or any website.
This site may provide links to other Internet sites only for the convenience of World Wide Web users. ACE is not responsible for the availability or content of these external sites, nor does ACE endorse, warrant or guarantee the products, services or information described or offered at these other Internet sites.
Although the information presented on this website is believed to be accurate at the time it is posted, this website could include inaccuracies, typographical errors or out-of-date information. This website may be changed at any time without prior notice.

