In this issue
- Medications reimbursement coverage in Canada
- What are “advanced therapies”?
- Reimbursement coverage challenges
- Patient support programs
- Living with lupus in Canada
- The Canadian drug review process
JointHealth™ insight Published May 2024


 Approximately 6 million people in Canada – 1 in in 5 adults – have arthritis. About 1 million of them live with inflammatory arthritis (IA), which describes the primary types of autoimmune diseases: rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. In more moderate, moderate to severe, and severe cases, they can be difficult to manage with conventional synthetic disease modifying medications or “csDMARDs” (such as methotrexate, sulfasalazine, leflunomide, or hydroxychloroquine). If a person with IA does not achieve optimal disease control on csDMARDs, then they are prescribed an advanced therapy.
Approximately 6 million people in Canada – 1 in in 5 adults – have arthritis. About 1 million of them live with inflammatory arthritis (IA), which describes the primary types of autoimmune diseases: rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. In more moderate, moderate to severe, and severe cases, they can be difficult to manage with conventional synthetic disease modifying medications or “csDMARDs” (such as methotrexate, sulfasalazine, leflunomide, or hydroxychloroquine). If a person with IA does not achieve optimal disease control on csDMARDs, then they are prescribed an advanced therapy.
What are “advanced therapies”?
Advances in therapy for patients with IA over the last 22 years have led to better disease management strategies and improved patient outcomes. The term “advanced therapies” is commonly used to describe medications (such as originator biologics, biosimilar biologics, and targeted small molecule) that treat some of the specific molecules that have been found to drive the inflammation, pain, and disability in inflammatory types of arthritis. Advanced therapies come in pill form or are given by intravenous injection or self-injected subcutaneously. They are grouped into two categories:
Advanced therapies come in pill form or are given by intravenous injection or self-injected subcutaneously. They are grouped into two categories:
Listening to you
We hope you find this information of use. Please tell us what you think by writing to us or emailing us at feedback@jointhealth.org. Through your ongoing and active participation, ACE can make its work more relevant to all Canadians living with arthritis.
Update your email or postal address
Please let us know of any changes by contacting ACE at feedback@jointhealth.org. This will ensure that you continue to receive your free email or print copy of JointHealth™ insight.
Arthritis Consumer Experts (ACE)
Who We Are
Arthritis Consumer Experts (ACE) and its team members acknowledge that they gather and work on the traditional, ancestral and unceded territory of the Coast Salish peoples -ʷməθkʷəy̓əm (Musqueam), Sḵwx̱wú7mesh (Squamish), and Səl̓ílwətaʔ/Selilwitulh (Tsleil-Waututh) Nations.
Arthritis Consumer Experts (ACE) operates as a non-profit and provides free research based education and information to Canadians with arthritis. We help (em)power people living with all forms of arthritis to take control of their disease and to take action in healthcare and research decision making. ACE activities are guided by its members and led by people with arthritis, scientific and medical experts on the ACE Advisory Board. To learn more about ACE, visit www.jointhealth.org
Disclosures
Over the past 12 months, ACE received grants-in-aid from: Amgen Canada, Arthritis Research Canada, Biosimilars Canada, Canadian Biosimilars Forum, Canadian Rheumatology Association, Eli Lilly Canada, JAMP Pharma, Novartis Canada, Organon Canada, Pfizer Canada, Sandoz Canada, Teva Canada, UCB Canada, the University of British Columbia and the University of Toronto.
ACE also received unsolicited donations from its community members (people with arthritis) across Canada.
ACE thanks funders for their support to help the nearly 6 million Canadians living with osteoarthritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and the many other forms of the disease.
Disclaimer
The material contained on this website is provided for general information only. This website should not be relied on to suggest a course of treatment for a particular individual or as a substitute for consultation with qualified health professionals who are familiar with your individual medical needs. Should you have any healthcare related questions, you should contact your physician. You should never disregard medical advice or delay in seeking it because of something you have read on this or any website.
This site may provide links to other Internet sites only for the convenience of World Wide Web users. ACE is not responsible for the availability or content of these external sites, nor does ACE endorse, warrant or guarantee the products, services or information described or offered at these other Internet sites.
Although the information presented on this website is believed to be accurate at the time it is posted, this website could include inaccuracies, typographical errors or out-of-date information. This website may be changed at any time without prior notice.


 Approximately 6 million people in Canada – 1 in in 5 adults – have arthritis. About 1 million of them live with inflammatory arthritis (IA), which describes the primary types of autoimmune diseases: rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. In more moderate, moderate to severe, and severe cases, they can be difficult to manage with conventional synthetic disease modifying medications or “csDMARDs” (such as methotrexate, sulfasalazine, leflunomide, or hydroxychloroquine). If a person with IA does not achieve optimal disease control on csDMARDs, then they are prescribed an advanced therapy.
Approximately 6 million people in Canada – 1 in in 5 adults – have arthritis. About 1 million of them live with inflammatory arthritis (IA), which describes the primary types of autoimmune diseases: rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. In more moderate, moderate to severe, and severe cases, they can be difficult to manage with conventional synthetic disease modifying medications or “csDMARDs” (such as methotrexate, sulfasalazine, leflunomide, or hydroxychloroquine). If a person with IA does not achieve optimal disease control on csDMARDs, then they are prescribed an advanced therapy.What are “advanced therapies”?
Advances in therapy for patients with IA over the last 22 years have led to better disease management strategies and improved patient outcomes. The term “advanced therapies” is commonly used to describe medications (such as originator biologics, biosimilar biologics, and targeted small molecule) that treat some of the specific molecules that have been found to drive the inflammation, pain, and disability in inflammatory types of arthritis.
 Advanced therapies come in pill form or are given by intravenous injection or self-injected subcutaneously. They are grouped into two categories:
Advanced therapies come in pill form or are given by intravenous injection or self-injected subcutaneously. They are grouped into two categories:
- Targeted synthetic disease modifying anti-rheumatic drugs or “tsDMARDs”
tsDMARDs are small molecules that target specific molecular structures in the body that drive an autoimmune disease. They are tested for safety and efficacy and approved for use by Health Canada. They include tofacitinib, baricitinib and apremilast. These are the “generic” or scientific compound name, not the “brand” name. - Biologic disease modifying anti-rheumatic drugs or “bDMARDs”
bDMARDs are large proteins that target specific components of the immune response that promote inflammation. They are given by intravenous injection or self-injected subcutaneously.
bDMARDS are divided into two sub-categories:
Originator biologic DMARDs (boDMARDs) are the first or original version of a biologic drug that a biosimilar is based on. As patents expire for originator biologics, other manufacturers may produce new, similar versions shown to be as safe and effective. Biosimilar biologic DMARDs (bsDMARDs) are biologic medications designed to be highly similar to an already existing approved originator biologic. Since the originator biologics are made from living cells, biosimilar biologics are highly similar but cannot be considered identical.
Originator biologic DMARDs and biosimilar biologic DMARDs are listed below using their scientific name followed by their “brand” name:
Originator biologic DMARDs Biosimilars biologic DMARDs abatacept (Orencia®) Not currently available adalimumab (Humira®) adalimumab (Arbrilada®, Amgevita®, Hadlima®, Hulio®, Hyrimoz®, Idacio®, Simlandi®, Yuflyma®) anakinra (Kineret®) Not currently available anifrolumab (Saphnelo®) Not currently available belimumab (Benlysta®) Not currently available bimekizumab (Bimzelx®) Not currently available canakinumab (Ilaris®) Not currently available certolizumab pegol (Cimzia®) Not currently available denosumab (Prolia®) Not currently available etancercept (Enbrel®) etanercept (Brenzys®, Erelzi®, Rymti®) golimumab (Simponi®) Not currently available guselkumab (Tremfya®) Not currently available infliximab (Remicade®) infliximab (Avsola®, Ixifi®, Renflexis®, Remsima®) ixekizumab (Taltz®) Not currently available rituximab (Rituxan®) rituximab (Riabni®, Riximyo®, Ruxience®, Truxima®) romosozumab (Evenity®) Not currently available sarilumab (Kevzara®) Not currently available secukinumab (Cosentyx®) Not currently available tocilizumab (Actemra®) Not currently available ustekinumab (Stelara®) ustekinumab (Jamteki®, Wezlana®)
Advanced therapies deliver life-saving value to those taking them, but also to healthcare systems and society. However, they come at higher prices which raises reimbursement challenges. The introduction of new advanced therapies has resulted in increased public and private drug program spending on inflammatory arthritis treatment. According to statistics from the Canadian Institute for Health Information, public drug program spending specifically on biologic medications reached $4.7 billion in 2022 (29.6% of total public drug program spending).1 For the 10th year in a row, anti-TNF type biologic medications used for inflammatory arthritis and Crohn’s disease made up the highest proportion of that public drug spending total. A closer look at public and private drug reimbursement plans in Canada
A closer look at public and private drug reimbursement plans in Canada
In Canada, a person with arthritis may receive reimbursement coverage for advanced therapy medications from public or private drug plans.
With each province and territory having its own healthcare system, there are various ways in which public drug reimbursement plans interact with private plans that impact access to medications to treat arthritis:- Canadians under 25 years of age generally have access to public coverage if they are not a beneficiary of their parents’ group plan.
- Among adults 25 to 64 years of age, a greater proportion are enrolled in a private, employer-based group plan, although employed individuals with no access to a group plan (as a primary member or beneficiary) and unemployed individuals must rely on a public plan or pay out-of-pocket. Some adults are even enrolled in both a public and private drug reimbursement plan; this can vary significantly by province or territory of residence.
- Canadians over 65 years of age are generally enrolled in a public plan rather than a private plan, although some are enrolled in both.
- Some Canadians may have no effective drug reimbursement coverage and pay the full cost for their prescription arthritis medications.
 Reimbursement coverage challenges
Reimbursement coverage challenges
The actual lived experience of people with arthritis and caregivers reveals significant challenges they face when trying to get reimbursement coverage for the medications they need. While serious and complex types of inflammatory arthritis affect nearly one million Canadians, their access to vital medications varies widely across the country.
These challenges can occur from the discrepancies in public and private drug reimbursement plan coverage across provinces and territories and across private health insurance plans. Federal, provincial, and territorial public drug reimbursement plans offer varying levels of coverage and decide who is covered and what the patient and plan pay based on medical need and income. Depending on where you live in Canada, the public drug plan might cover different arthritis medicines. This means that not everyone in Canada has the same access to arthritis medicines even with the same type of plan, which raises equitability issues. Some people with arthritis in some provinces or territories must pay more for their arthritis medications than people in other provinces or territories living with the same disease.
Access under private drug reimbursement plans also varies depending on which plan you have, because different plans cover different things.
Getting drug reimbursement coverage for more costly biologics (originator and biosimilar) is even more challenging and often requires going through a special administrative process and extra paperwork. Some medically necessary biologic medications are virtually unaffordable for those without a private drug reimbursement plan, due to high out-of-pocket costs. People with arthritis face out-of-pocket costs if a prescribed medication does not line up properly with a public or private drug plan’s official list of approved medications for reimbursement coverage, called the formulary (for example, full cost if medication is not on the formulary; partial cost if there is a co-payment or deductible).
 The impact of reimbursement coverage challenges for people with arthritis
The impact of reimbursement coverage challenges for people with arthritis
Even with coverage, getting reimbursed for prescription medications can be complex. An ACE National Survey on Arthritis Medications Reimbursement for People with Arthritis found that almost 20% of respondents found the process difficult. Black, Indigenous, and other people of colour (BIPOC) who responded to the Survey reported facing these issues at a higher rate.
The Survey also uncovered reimbursement coverage shortfalls. Public plans, while helpful, might not always provide adequate coverage. Nearly 40% of survey respondents with public plans reported insufficient coverage for their medications. This can leave people dealing with chronic diseases with significant out-of-pocket costs in the form of deductibles or co-payments.
These challenges are causing disease progression and more difficulties with daily activities and forcing a significant percentage of respondents to make tough choices on how to obtain and stay on their life-changing medications. In the Survey, ACE asked respondents what inadequate reimbursement coverage for prescribed arthritis medications forced them to do. Here are how respondents answered from a list of options:- Seek reimbursement coverage from pharmaceutical company: 13%
- Take less of the prescribed medication (for example skipping a dose or splitting pills): 8%
- Not fill your new prescription: 8%
- Not go on vacation: 6%
- Start taking a different medication: 5%
- Fill some prescription medications over others: 5%
- Borrow money to pay for medications: 5%
- Not renew your prescription: 3%
- Stop taking your prescribed medication: 3%
- Take medications prescribed for someone else: 1%

Addressing the challengesCall to action
Share your experience and be a part of ACE advocacy
ACE advocacy includes listening carefully to the issues, concerns, and experiences that our members share with us. For example, the issue of how the voices of racial minorities are often underrepresented or excluded in health research. We then take the valuable information gathered from underrepresented communities and create the messages and policy recommendations and deliver our arthritis story or “ask” to elected officials and policymakers.
What has been your experience dealing with your current public or private drug reimbursement plan? Your feedback will help ACE when it meets with provincial, territorial, or private health insurance plan administrators and decision-makers to discuss how to improve reimbursement coverage.
For public and private drug reimbursement plans to effectively review and list new medications and provide reimbursement access to treatment options, it is important for them to be aware of the unmet needs of people dealing with arthritis, while at the same time, ensuring patients can afford the medications they need.
Biologic medicines are life-changing treatments for people living with inflammatory arthritis. They also continue to be a growing budget pressure for public and private drug reimbursement plans. Today, public drug plans are cost-managing formularies by restricting coverage for existing medications, delaying access to new medications and, at times, failing to address unmet treatment needs.
Private insurers and pharmacy benefit managers have implemented a wide variety of cost containment programs and tools to manage plan sponsors’ drug plan costs, including:
- Case management of drug claims: insurer reviews physician’s proposed treatment plan to ensure it’s “reasonable,” identifies alternative treatments; monitors adherence; limits payments
- Preferred pharmacy networks: “Requiring” consumers to purchase from specific pharmacies that offer medications at lower prices

Public coverage of arthritis medications varies across provinces and territories
People living with inflammatory arthritis continue to face hurdles related to the cost, coverage, and accessibility to advanced therapies depending on where they live, underscoring the need for ongoing advocacy to improve access to these life-changing treatments.
Over the past 18 years, Arthritis Consumer Experts’ Arthritis Medications Report Card has been integral to developing and implementing new arthritis health policy by making decision-making transparent and by highlighting inequity in medication reimbursement towards ensuring the treatments are more affordable and accessible.
Public drug plan policies that respond to the needs of people living with arthritis at national, provincial, and territorial government levels can change their lives. The Report Card is designed to help Canadians monitor changes to public drug reimbursement plans and evaluate where their province ranks in terms of providing coverage for medications approved for inflammatory arthritis such as rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis and juvenile idiopathic arthritis.
Arthritis relief: Understanding the role of patient support programs in Canada for inflammatory arthritis patientsCall to action
Your voice is powerful
Individuals living with inflammatory arthritis need affordable and prompt access to proven and effective medications that treat their underlying disease process and help them maintain their quality of life.
By helping policymakers understand what is important to people who know arthritis firsthand, you can shape their decision-making. If a public drug plan isn’t meeting your specific needs, ACE invites your feedback so that we can continue our decades long work to fight for equitable reimbursement for you, and for our entire community.
For some living with inflammatory forms of arthritis, day to day life can be painful and a real struggle so accessing necessary treatments shouldn't be. To assist people living with complex chronic diseases like inflammatory arthritis, pharmaceutical companies offer patient support programs to assist with much of the initial paperwork required to determine whether a person’s public or private drug reimbursement plan will provide coverage for the medication. Patient programs are designed to provide coordination between the physician’s office – at the time the prescription is handed to a patient – and for the entirety of a person’s time on their medication and while enrolled in the program. What should a person prescribed an advanced therapy expect from the patient support program?
What should a person prescribed an advanced therapy expect from the patient support program?
Once a rheumatologist prescribes a specific advanced therapy to a patient, the patient program coordinator can reach out to them to begin the enrollment process. Nearly all advanced therapies used to treat inflammatory forms of arthritis have a patient support program.
At the same time the patient support program coordinator begins processing paperwork, they also reach out to the person prescribed the medication to do an initial needs assessment. Based on the patient’s specific needs, the coordinator recommends services available in the program to customize it. Patient support program services vary from pharmaceutical company to pharmaceutical company, but all offer help with getting reimbursement coverage from the person’s public or private insurer, information and education resources, nursing support (for things like self-injection training) and even financial assistance in dire emergencies.
The patient support programs can also help patients navigate the medication reimbursement process, including:- pre-authorization requirements for prescription medications
- financial assistance
- generic substitution
- biosimilars switching
- appeals process if medication reimbursement coverage is declined
- insurance plan limitations or exclusions
- specialist referrals
- medication management support (training on how to take the medication, for example, injection training)
- care coordination (interacting with different health care providers, managing appointments and treatments)
- disease management programs (access to specialized nurses, nutritionists)
- educational resources and counseling services (diet, exercise, mental health)
- health and wellness plans for arthritis
- virtual care services (online meetings with health care professionals)
- digital health tools (online platforms and apps to access health records or medication schedules, monitor symptoms)
Originator biologic DMARDs (boDMARDs)
Medication Patient support program name Link abatacept (Orencia®) ORENCIA On Call Program website adalimumab (Humira®) AbbVie Care Support Program website anakinra (Kineret®) KINERET On Track™ Patient Support Program website anifrolumab (Saphnelo®) Connect 360 Patient Support Program website belimumab (Benlysta®) Monarch Patient Support Program website bimekizumab (Bimzelx®) UCBCares® patient support program website canakinumab (Ilaris®) Ilaris Companion website certolizumab pegol (Cimzia®) UCBCares® patient support program website denosumab (Prolia®) Amgen ProVital Program website etanercept (Enbrel®) Amgen Enliven® Services website golimumab (Simponi®) Janssen BioAdvance® program website guselkumab (Tremfya®) Janssen BioAdvance® program website infliximab (Remicade®) Janssen BioAdvance® program website ixekizumab (Taltz®) LillyPlus® program website rituximab (Rituxan®) Roche OnCare™ website romosozumab (Evenity®) Amgen ProVital Program website sarilumab (Kevzara®) Sanofi Mobilize website secukinumab (Cosentyx®) Novartis XPOSE® program website tocilizumab (Actemra®) Roche JointEffort® website ustekinumab (Stelara®) Janssen BioAdvance® program website  Biosimilar biologic DMARDs (bsDMARDs)
Biosimilar biologic DMARDs (bsDMARDs)
Medication Patient support program name Link adalimumab (Abrilada®) PfizerFlex Patient Support Program website adalimumab (Amgevita®) Amgen Entrust™ Patient Support Services website adalimumab (Hadlima®) HARMONY BY ORGANON™ Patient Support Program website adalimumab (Hulio®) Biocon Patient Support Program website adalimumab (Hyrimoz®) SANDOZ Patient Support Program website adalimumab (Idacio®) KabiCare® Patient Support Program website adalimumab (Simlandi®) JAMP Care™ patient support program website adalimumab (Yuflyma®) Celltrion CONNECT™ Patient Support Program website etanercept (Brenzys®) HARMONY BY ORGANON™ Patient Support Program website etanercept (Erelzi®) SANDOZ Patient Support Program website etanercept (Rymti®) Lupin Pharma Canada website infliximab (Avsola®) Amgen Enliven® Services website infliximab (Inflectra®) PfizerFlex Patient Support Program website infliximab (Renflexis®) HARMONY BY ORGANON™ Patient Support Program website infliximab (Remsima®) Celltrion CONNECT™ Patient Support Program website rituximab (Riabni®) Amgen Entrust™ Patient Support Services website rituximab (Riximyo®) SANDOZ Patient Support Program website rituximab (Rituxan®) Roche JointEffort® website rituximab (Ruxience®) PfizerFlex Patient Support Program website rituximab (Truxima®) Truxima Teva Support Solutions® website ustekinumab (Jamteki®) JAMP Care™ patient support program website ustekinumab (Wezlana®) Wezlana® Enliven® Services website
Targeted synthetic DMARDs (tsDMARDs)
Medication Patient support program name Link tofacitinib (Xeljanz®) PfizerFlex Patient Support Program website baricitinib (Olumiant®) LillyPlus® program website upadacitinib (Rinvoq®) AbbVie Care Support Program website apremilast (Otezla®) ez Start Patient Support Program website  Living with lupus in Canada: A struggle for access to important advanced therapies
Living with lupus in Canada: A struggle for access to important advanced therapies
Many Canadians with systemic lupus erythematosus (SLE) face a frustrating challenge: getting the life-saving medications they need. This can depend on where they live and what medication insurance coverage they have, public or private. Even though rheumatologists and arthritis patient organizations have been pushing for better reimbursement coverage, it's happening too slowly or not at all. This lack of appropriate access to advanced therapies hurts people with lupus, the healthcare system, and the economy.
Systemic lupus erythematosus is an autoimmune disease that affects about 1 in 2,000 Canadians. While we do not know what causes SLE, many scientists believe that it develops in response to a combination of factors both inside and outside the body, including hormones, genetics, and environment. The disease occurs when the body’s immune system begins to malfunction and attack healthy tissue in various parts of the body, causing inflammation and damage. Affected areas can include the skin, joints, muscles, kidneys, lungs, heart, blood vessels, and brain. Lupus nephritis is one of the most serious complications of SLE and is seen to some degree in about 60% of SLE patients. It occurs when the immune system mistakenly attacks the kidneys, leading to inflammation and possibly to organ damage.
Like some other types of inflammatory arthritis, women are more likely to develop it than men. It is also more common in Black, Indigenous, and people of color (BIPOC) communities. The complexity of lupus, with its unknown exact cause and potential triggers ranging from genetics to environmental factors, makes its management particularly challenging. Early and effective treatment is crucial to prevent long-term damage from the disease.
In 2011, Health Canada approved a medication called belimumab (Benlysta®), which offered hope for many lupus patients. Unfortunately, a government agency that reviews medications, the Canadian Agency for Drugs and Technologies in Health (CADTH) gave it a negative recommendation which meant most public drug plans would not list it except as an exceptional drug in Quebec. Ten years later, Health Canada has recently approved another new SLE advanced therapy called anifrolumab (Saphnelo®), which received a positive CADTH review and recommendation to reimburse. In the past six months, anifrolumab was listed on the Alberta, Quebec, Ontario, New Brunswick, Manitoba, Newfoundland & Labrador, Nova Scotia, and Non-insured Health Benefits for First Nations and Inuit public drug plans. Offering further hope for the long-time unmet needs of people with lupus, a new listing for belimumab (Benlysta®) for lupus nephritis is being considered for coverage by public drug plans after receiving a positive CADTH review.
There is cautious optimism that more Canadians will have access to these medications in the future. However, this progress also highlights a gap. Many people cannot afford these drugs without public or private drug plan reimbursement coverage. A recent survey showed that 1 in 5 Canadians lack any private insurance to help pay for medications, with higher rates among seniors, immigrants, and racialized groups.2 Many people with lupus fall into this category.
Another barrier to reimbursement coverage is the differences in criteria between public drug plans. Someone with lupus, for example, lucky enough to be on a lupus advanced therapy in Quebec where the public drug plan covers their medication move to a province that doesn't provide coverage, like British Columbia, could lose access to treatment and experience a worsening of their disease.
Arthritis Consumer Experts, along with Lupus Canada and rheumatology leaders from across the country have formed a Lupus Working Group and are working hard to advocate for more equitable access to advanced therapies for Canadians lupus patients. Drug plan listings of lupus advanced therapies for reimbursement coverage are a life-saving moment for people threatened by the disease, providing timely access to a safe, effective biologic treatment and offering patients a chance at a better quality of life.
People with lupus deserve nothing less.
Meeting with elected officials and policy makers
Arthritis Consumer Experts elevates the needs of people with arthritis by meeting regularly with elected officials and policy makers to educate them on issues of importance to the arthritis community, including public drug reimbursement plans. This work may be accomplished independently, by partnering with other rheumatology or patient groups or organizations, or through coalitions like the Lupus Advocacy Working Group, described above. Other examples include ACE’s partnership with the Ontario Rheumatology Association on improving the models of care in Northern Ontario, or our leadership in forming the Arthritis Community Learning Circle and Arthritis Call to Action website to walk forward on the path towards Truth and Reconciliation, or our work in coalition with Arthritis Society Canada and 18 other arthritis community partners to develop and implement the Arthritis Action Plan. The Canadian drug review process: are Canadians with arthritis represented?
The Canadian drug review process: are Canadians with arthritis represented?
In Canada, the drug review process takes place in three stages:- Federal Government
- Health Canada reviews drug safety, effect of the drug and quality of the manufacturing process
- National Review
- Canadian Agency for Drugs and Technologies in Health (CADTH) Common Drug Review (CDR) reviews how well the drug works when compared to similar drugs that are used to treat the same disease and whether the drug provides value for money.
- Pan-Canadian Pharmaceutical Alliance (PcPA) negotiates on behalf of public drug plans with drug manufacturer to achieve greater value for drug.
- Public Drug Plan carries out its own review before deciding to list a drug for reimbursement coverage building on the work completed by Health Canada, CADTH/CDR and PcPA
 The power of arthritis patient feedback
The power of arthritis patient feedback
Besides considering a medication’s safety, how well it works, how much it costs, and how it compares to other similar drugs as part of their decision-making on which medications are covered for reimbursement, CADTH and public drug plans collect feedback provided by people with experience using the medication under review and with the disease it would treat. Through surveys, patients, caregivers, and patient groups can share their experiences and opinions about the medications under review, contributing valuable insights that go beyond clinical data. Arthritis Consumer Experts advocates for the arthritis patient community in Canada by responding to public drug plans’ calls for patient inputs. Our submissions help inform policy decisions that affect the lives of people living with arthritis. Since its inception in 1999, ACE has delivered more patient input submissions than any other patient group organization in Canada.
Arthritis Consumer Experts advocates for the arthritis patient community in Canada by responding to public drug plans’ calls for patient inputs. Our submissions help inform policy decisions that affect the lives of people living with arthritis. Since its inception in 1999, ACE has delivered more patient input submissions than any other patient group organization in Canada.
Whenever CADTH or public drug plans issue calls for patient input, ACE reaches out to the arthritis community for patient input about their lived experience and submits these inputs anonymously to health policymakers.
Arthritis Consumer Experts’ Patient Input Process
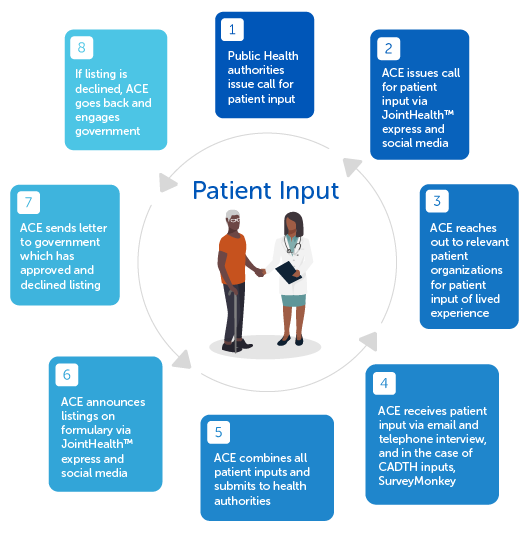 Currently, ACE is focusing on representing those living with axial spondyloarthritis (AS) and psoriatic arthritis (PsA) as part of the patient input process underway for a new medication – bimekizumab (Bimzelx®) – that could help address their unmet needs. As part of its submission process, ACE presents AS and PsA patient views on how bimekizumab (Bimzelx®) could enable them to engage in daily activities with less discomfort and enhance their quality of life.
Currently, ACE is focusing on representing those living with axial spondyloarthritis (AS) and psoriatic arthritis (PsA) as part of the patient input process underway for a new medication – bimekizumab (Bimzelx®) – that could help address their unmet needs. As part of its submission process, ACE presents AS and PsA patient views on how bimekizumab (Bimzelx®) could enable them to engage in daily activities with less discomfort and enhance their quality of life.
By submitting detailed feedback on treatments such as bimekizumab (Bimzelx®), ACE ensures that the voices of those living with arthritis are heard, influencing policies and practices that directly affect their lives. This process not only aids in the evaluation of new treatments but also highlights the importance of considering the patient's lived experience alongside traditional clinical assessments, ultimately ensuring that treatment options listed for reimbursement coverage align closely with the needs and realities of those they aim to serve.Targeting IL-17A and IL-17F in AS and PsA
Bimekisumab (Bimzelx®)’s mechanism of action (i.e. how a medication produces an effect in the body) targets IL-17A and IL-17F, two small proteins involved in the body's inflammatory response, which are key factors in the progression of AS and PsA. Clinical trials have shown that bimekizumab (Bimzelx®) can significantly improve symptoms in patients with AS and PsA, including joint pain, stiffness, and mobility.
For AS patients, inhibiting these proteins can reduce inflammation and bone formation, addressing the root of the disease. PsA patients benefit from a comprehensive reduction in joint inflammation and skin symptoms, leading to an overall decrease in disease activity.
Call to action
It's important for Canadian arthritis patients to share their experiences during public drug plan reviews of new medications under consideration for reimbursement coverage. Patient input helps make sure medications meet their needs, work well in daily life, and cater to a diverse patient population, including those with varying levels of disease severity, other illnesses or diseases, or other factors like age or ethnicity, which may affect treatment efficacy. This makes healthcare policies clear and focused on patients.
Your voice and personal experiences can shape policymakers’ decisions on the next arthritis medication being considered for reimbursement coverage. To receive our calls for patient input submissions, please subscribe to ACE’s JointHealth™ express newsletter. References
References
- Canadian Institute for Health Information. Prescribed Drug Spending in Canada, 2022. CIHI; 2023. Infographic https://www.cihi.ca/en/prescribed-drug-spending-in-canada-2023
- Cortes K. Smith L. Pharmaceutical access and use during the pandemic. November 2, 2022. Available at https://www150.statcan.gc.ca/n1/en/catalogue/75-006-X202200100011. Accessed February 5, 2024.
Listening to you
We hope you find this information of use. Please tell us what you think by writing to us or emailing us at feedback@jointhealth.org. Through your ongoing and active participation, ACE can make its work more relevant to all Canadians living with arthritis.
Update your email or postal address
Please let us know of any changes by contacting ACE at feedback@jointhealth.org. This will ensure that you continue to receive your free email or print copy of JointHealth™ insight.
Arthritis Consumer Experts (ACE)
Who We Are
Arthritis Consumer Experts (ACE) and its team members acknowledge that they gather and work on the traditional, ancestral and unceded territory of the Coast Salish peoples -ʷməθkʷəy̓əm (Musqueam), Sḵwx̱wú7mesh (Squamish), and Səl̓ílwətaʔ/Selilwitulh (Tsleil-Waututh) Nations.
Arthritis Consumer Experts (ACE) operates as a non-profit and provides free research based education and information to Canadians with arthritis. We help (em)power people living with all forms of arthritis to take control of their disease and to take action in healthcare and research decision making. ACE activities are guided by its members and led by people with arthritis, scientific and medical experts on the ACE Advisory Board. To learn more about ACE, visit www.jointhealth.org
Disclosures
Over the past 12 months, ACE received grants-in-aid from: Amgen Canada, Arthritis Research Canada, Biosimilars Canada, Canadian Biosimilars Forum, Canadian Rheumatology Association, Eli Lilly Canada, JAMP Pharma, Novartis Canada, Organon Canada, Pfizer Canada, Sandoz Canada, Teva Canada, UCB Canada, the University of British Columbia and the University of Toronto.
ACE also received unsolicited donations from its community members (people with arthritis) across Canada.
ACE thanks funders for their support to help the nearly 6 million Canadians living with osteoarthritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and the many other forms of the disease.
Disclaimer
The material contained on this website is provided for general information only. This website should not be relied on to suggest a course of treatment for a particular individual or as a substitute for consultation with qualified health professionals who are familiar with your individual medical needs. Should you have any healthcare related questions, you should contact your physician. You should never disregard medical advice or delay in seeking it because of something you have read on this or any website.
This site may provide links to other Internet sites only for the convenience of World Wide Web users. ACE is not responsible for the availability or content of these external sites, nor does ACE endorse, warrant or guarantee the products, services or information described or offered at these other Internet sites.
Although the information presented on this website is believed to be accurate at the time it is posted, this website could include inaccuracies, typographical errors or out-of-date information. This website may be changed at any time without prior notice.

