In this issue
- Background
- Who were the Survey respondents?
- Reimbursement coverage for arthritis medications
- Do respondents know what medicines they take?
- What were specific challenges with accessing reimbursement coverage for arthritis medication?
- Comparing different patient populations' experiences
- A deeper look at reimbursement access and affordability
- Getting the information and support needed to navigate the arthritis medication reimbursement process
- Addressing the challenges to reimbursement access
- How do you get involved?
JointHealth™ insight Published July/August 2021

 Although many Canadians have access to public or private drug insurance, the patchwork of drug plans in Canada creates inequities in access to medications that can lead to reduced treatment and treatment delays, exposes households and businesses to considerable financial burden, and isolates the management of prescription drugs from other key components of the Canadian healthcare system.
Although many Canadians have access to public or private drug insurance, the patchwork of drug plans in Canada creates inequities in access to medications that can lead to reduced treatment and treatment delays, exposes households and businesses to considerable financial burden, and isolates the management of prescription drugs from other key components of the Canadian healthcare system.
Listening to you
We hope you find this information of use. Please tell us what you think by writing to us or emailing us at feedback@jointhealth.org. Through your ongoing and active participation, ACE can make its work more relevant to all Canadians living with arthritis.
Update your email or postal address
Please let us know of any changes by contacting ACE at feedback@jointhealth.org. This will ensure that you continue to receive your free email or print copy of JointHealth™ insight.
Arthritis Consumer Experts (ACE)
Who We Are
Arthritis Consumer Experts (ACE) operates as a non-profit and provides free research based education and information to Canadians with arthritis. We help (em)power people living with all forms of arthritis to take control of their disease and to take action in healthcare and research decision making. ACE activities are guided by its members and led by people with arthritis, scientific and medical experts on the ACE Advisory Board. To learn more about ACE, visit www.jointhealth.org
Disclosures
Over the past 12 months, ACE received grants-in-aid from: Arthritis Research Canada, Amgen Canada, Canadian Biosimilars Forum, Canadian Institutes of Health Research, Canadian Rheumatology Association, Eli Lilly Canada, Fresenius Kabi Canada, Gilead Sciences Canada, Hoffman-La Roche Canada Ltd., Knowledge Translation Canada, Merck Canada, Novartis Canada, Pfizer Canada, Sandoz Canada, Sanofi Canada, St. Paul's Hospital (Vancouver), Teva Canada, UCB Canada, and the University of British Columbia.
ACE also received unsolicited donations from its community members (people with arthritis) across Canada.
ACE thanks funders for their support to help the nearly 6 million Canadians living with osteoarthritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and the many other forms of the disease.
Disclaimer
The material contained on this website is provided for general information only. This website should not be relied on to suggest a course of treatment for a particular individual or as a substitute for consultation with qualified health professionals who are familiar with your individual medical needs. Should you have any healthcare related questions, you should contact your physician. You should never disregard medical advice or delay in seeking it because of something you have read on this or any website.
This site may provide links to other Internet sites only for the convenience of World Wide Web users. ACE is not responsible for the availability or content of these external sites, nor does ACE endorse, warrant or guarantee the products, services or information described or offered at these other Internet sites.
Although the information presented on this website is believed to be accurate at the time it is posted, this website could include inaccuracies, typographical errors or out-of-date information. This website may be changed at any time without prior notice.



Arthritis Consumer Experts (ACE) conducted a national Survey in April 2021 to identify inequities and gaps in public and private drug plans – who is experiencing them and what some of the challenges are in getting reimbursement for needed life-changing arthritis medications.

Background
Under the Canada Health Act, prescription drugs administered in Canadian hospitals are provided at no cost to the patient. Outside of the hospital setting, most Canadians have some access to insurance coverage for prescription drugs through public and/or private drug plans.
The federal, provincial and territorial governments offer varying levels of coverage and decide who is covered and what the patient and plan pays. The publicly funded drug programs generally provide drug plan coverage for those most in need, based on age, income, and medical condition. Additionally, federal public drug plans provide coverage for veterans, First Nations and Inuit individuals, federally incarcerated offenders, the Royal Canadian Mounted Police, and the military.
Many Canadians and their family members have drug coverage linked to employment and private drug plans through an extended health benefit plan. Some Canadians may have no effective drug coverage and pay the full cost of prescription drugs.
Methodology
ACE conducted a 30-question online Survey between April 12, 2021 and April 30, 2021 of people with a physician- diagnosed arthritis in English and French. Respondents answered questions about the accessibility and affordability of reimbursement coverage for prescribed arthritis medications. Respondents required internet access to complete the Survey. Survey findings were analysed in aggregate (including incomplete Survey responses).
Who were the Survey respondents?
Arthritis Consumer Experts received 319 responses, including 249 in English and 70 in French (69 were Quebec residents).
Respondents were patients living with different forms of arthritis, including osteoarthritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, lupus, juvenile idiopathic arthritis, fibromyalgia, Sjögren’s syndrome, scleroderma, and Behcet’s disease. The three most common forms of arthritis among respondents were rheumatoid arthritis (accounting for almost half of the respondents), osteoarthritis (1 in 10 or 14%), and ankylosing spondylitis (1 in 10 or 9%).
Forty-five per cent (5 in 10) of the respondents have been living with arthritis for over 10 years, while 14% (1 in 10) have been living with arthritis between 6-10 years and 41% (4 in 10) have been living with arthritis for 5 years or less.
In terms of gender, 8 in 10 (79%) of respondents were women, 2 in 10 (20%) were men, 0.33% respondents considered themselves as non-binary, 0.66% respondents selected other, and 0.66% respondents preferred not to answer this question.
A majority of respondents travel 2 times per year to see their rheumatologist (a little over 3 in 10 or 33%); followed by 3 in 10 or 30% who travel once a year to see their rheumatologist. One in 10 or 15% travel 3 times per year to see their rheumatologist, while 2 in 10 or 22% travel 4 or more times per year to see their rheumatologist.
Nearly 40% of respondents travel 1-10km to see their rheumatologist, followed by: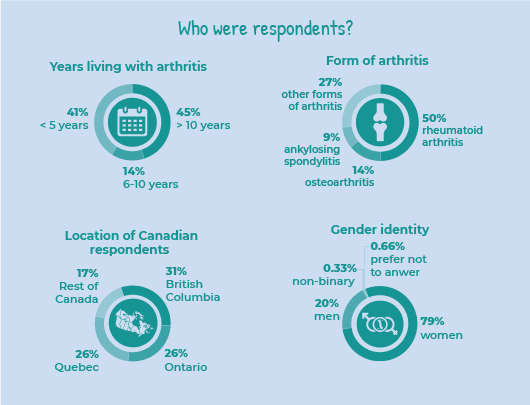
Reimbursement coverage for arthritis medications
To identify the gaps in reimbursement coverage for arthritis medications in Canada, we asked Survey respondents to tell us how their arthritis prescription medications get reimbursed (paid for):
How easy or difficult is it to navigate the reimbursement access process?
Two in 10 or 19% of respondents reported that the reimbursement access process was not easy and straightforward. While close to 7 in 10 or 68% of respondents strongly agreed or agreed that the process of getting reimbursement through public or private drug plans for their arthritis medication has generally been easy and straightforward.
More than 1 in 10 or 14% of respondents found it was neither easy nor tough.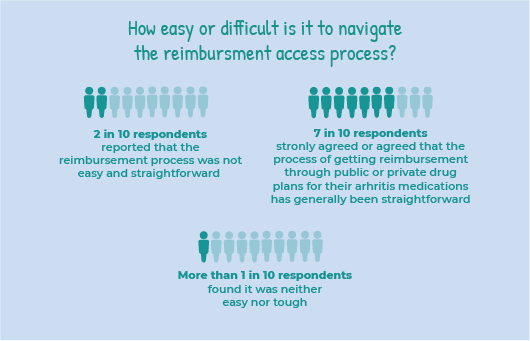

What respondents told us:
“A map to guide patients through options for coverage (private, pharmacare, drug companies). Personally, my ‘coverage’ has changed numerous times depending on my own life stage – dependent, student, employee of small/medium/large businesses. Each life change, and each medication change, has come with a change in coverage.”
“The approval process for my medication can be somewhat confusing as several parties are involved - my rheumatologist, the provincial Medicare program, the provincial Seniors Pharmacare program, the drug company and of course the pharmacy. So it can be hard to keep track of who does what in the processes.”
“I think to have my medication for pain covered by Non-Insured Health Benefits for Indigenous (NIHB), I pay for my over-the-counter medication monthly. I have low income and not on social assistance.”
What were specific challenges with accessing reimbursement coverage for arthritis medication?
In the Survey, ACE asked respondents to share what specific difficulties they experienced when accessing coverage for their prescription arthritis medication. They could select multiple answers from a list of options. The options included:
What respondents told us:
“I have to pay out of pocket until I meet my deductible for my private health insurance. The private health insurance pays until I meet my pharmacy requirements deductible. My husband retired last year and now my deductible has increased. With the biologic plus other medication I take, the monetary burden for my necessity medication has gone up significantly.”
“There was a great deal of stress because I knew I couldn't pay and yet I knew that without the drugs, I couldn't walk or function.”

Comparing different patient populations' experiences
A statistically significant higher number of respondents who identified as French reported that they did not experience any difficulties when it comes to getting coverage for their prescription arthritis medications. Specifically, 73% (7 in 10) of French respondents compared to nearly half or 47% (5 in 10) of English respondents reported not experiencing any difficulties.
There were also sharp differences with challenges in accessing reimbursement coverage for arthritis medication for black, Indigenous, and people of colour (BIPOC) respondents compared to the Survey findings for the general population.
Respondents who identified as BIPOC were 3 times more likely to report the forms they needed to fill out for reimbursement were confusing. Specifically, 29% (3 in 10) of BIPOC respondents reported that the forms they needed to fill out for reimbursement were confusing compared to 9% (1 in 10) white respondents. In addition, there were 4 times more BIPOC respondents who indicated there were too many forms to fill out with 24% of BIPOC respondents compared to 6% of white respondents. These findings underscore that the reimbursement forms may be too complicated to understand for people who do not speak English or French as a first language and the possibility of a lack of available translation services at various stages of the application process for medications reimbursement.

A deeper look at reimbursement access and affordability
ACE’s Survey findings showed a similar percentage of respondents on public and private drug plans who reported on how adequately their drug plan paid for arthritis medications and on their plan affordability.
Findings from respondents who have private drug plan coverage
Out of the 154 respondents who have private drug plan coverage, 70% (7 in 10) of respondents reported their private drug plan pays for most, or all, of their arthritis medications. While most respondents reported receiving adequate reimbursement coverage, 30% (3 in 10) of respondents reported their private drug plan did not adequately pay for their arthritis medications.
In terms of affordability, 54% (5 in 10) of respondents who identified as having private drug plan coverage reported their portion of private drug plan premium is affordable, while 19% (2 in 10) reported their portion as not affordable. Around 16% (2 in 10) of respondents who identified as having private drug plan coverage said their employer pays the entire premium of their private drug plan. 11% (1 in 10) indicated they do not know/am not sure if their insurance premium is affordable. It is important to note here that 48 respondents reported having both a public and a private drug plan coverage.
Findings from respondents who have public drug plan coverage
Out of the 136 respondents who have public drug plan coverage, 65% (over 6 in 10) reported that their public drug plan pays for most, or all, of their arthritis medications. While most respondents reported their public drug plan provided adequate reimbursement coverage, 35% (4 in 10) reported their public drug plan does not adequately pay for their arthritis medications. It is important to note here that 48 respondents reported having both a public and a private drug plan coverage.

The impact of poor reimbursement coverage for arthritis medications
In the Survey, ACE asked respondents what poor reimbursement coverage for prescribed arthritis medications forced them to do. They could select multiple answers from a list of options. The options included:
What respondents told us:
“Delayed purchasing a home due to cash flow being spent on meds rather than being saved.”
“I wait longer between treatments, so experience higher pain for a couple of months.”
“I have private coverage until I am 65, I don’t know what it will be like when I no longer have that private insurance in another 3 and a half years from now. I do worry about that, and if it will be affordable at that time or not. “
“We have just enough to make it each month. Some weeks are pretty tight on food to make sure I get my pills.”
Getting the information and support needed to navigate the arthritis medication reimbursement process
ACE asked respondents if they felt they had the information and support needed to understand reimbursement option and easily navigate the reimbursement process. Six in 10 or 61% of respondents reported they “strongly agree” or “agree” that they have the information and support needed.
By comparison, 17% of respondents said they “strongly disagree” or “disagree” that they have the information and support needed to understand reimbursement options and easily navigate the reimbursement process. According to respondents, here are some of the ways to help them get the information and support they need to better understand the reimbursement process:
What respondents told us:
“It is very confusing for young people who age out of the parents' coverage (I aged out of my mother's private coverage when I was no longer a full-time student) but don't have their own coverage yet. It will be even more confusing and worrisome when I turn 25 and am no longer covered by my dad's private coverage or OHIP+.”
“The extent of support I've received is the flyers that come with various biologic injections explaining whom to contact for manufacturers' support programs. The nurses who have provided these have, generally, not explaining any of the particulars, which vary by manufacturer. The ability to work with a specific pharmacist or nurse across medications (I'm sure I'm not the only arthritis patient who has tried many medications and often has to switch prescriptions) who has extensive and up-to-date training in seeking reimbursements would be very helpful. “
“I have no idea who when and how my new medication is going to get paid for. I have no idea if my old medication is good to be continued to pay for. One agency points the finger to the next. So I am left in the dark. Why can’t there be one agency to help me figure out my costs, help me with paperwork in a timely manner and send me regular (at least once a year) communication (either by phone, email or mail) regarding how my coverage is setup and if there’s any changes.”

Discussion
Addressing the challenges to reimbursement access
For government regulators and public and private drug plans to effectively review and approve new therapies and provide reimbursement access to treatment options, it is important for them to be aware of the unmet needs of arthritis patients; while at the same time, ensuring patients can afford the medicines they need.
Advanced therapies to treat inflammatory arthritis, such as biologics (originator and biosimilar) or targeted small molecule medications, deliver value both medically and socially. They also cost significantly more than other more conventional medicines. Over time, the introduction of new advanced therapies has resulted in increased public and private drug program spending on inflammatory arthritis treatment. For example, in 2018, biologic medicines accounted for less than 2% of prescribed drugs in Canada, yet the costs associated with them represented nearly 30% of Canada’s total prescription drug costs.2
Today, public drug plans are cost-managing formularies by restricting coverage for existing medications, delaying access to new medications and, at times, failing to address unmet treatment needs. Private insurers and pharmacy benefit managers have implemented a wide variety of cost containment programs and tools to manage plan sponsors’ drug plan costs, including case management and preferred pharmacy networks.
Survey findings show that reimbursement coverage accessibility and affordability for prescribed arthritis medications are causing hardship and forcing a significant percentage of respondents to make difficult choices to obtain and stay on their life-changing medications.

Advocating for improved reimbursement access
The findings from ACE’s National Survey on Arthritis Medications Reimbursement for People Living with Arthritis expose the need to amplify patient needs and priorities throughout the regulatory approval and reimbursement access process.
That requires patient organizations like ACE and individual patients to take action to support expanding access to arthritis medications. Patients can get involved by advocating for policies that move new medications through the most efficient review process, while still upholding strict safety and efficacy standards, and receive timely, equitable reimbursement coverage.
People with arthritis are the experts living with the disease: How do you get involved?
Patient input
ACE advocates for the Canadian patient community by responding to call for patient inputs. These inputs help inform policy decisions that affect the lives of Canadians living with arthritis. ACE has delivered more patient input submissions since its inception in 1999 than any other patient group organization in Canada.
Whenever public health authorities issue calls for patient input, ACE reaches out to the arthritis community for patient input of lived experience and submits these inputs to health authorities. To receive our calls for patient input submission, please subscribe to our JointHealth™ express newsletter.
How does your province or territory measure up?
Are there gaps in reimbursement coverage for medications approved for inflammatory arthritis such as rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis and juvenile idiopathic arthritis in your province or territory? ACE’s Arthritis Medications Report Card is designed to help Canadians measure their province or territories’ performance.
If your jurisdiction doesn’t measure up, ACE encourages you to write and speak to your elected provincial or federal representative about the lack of equitable reimbursement access and patient/physician choice in treating inflammatory forms of arthritis. Please go to ACE’s Advocacy page on our website where you can find information on how to contact your elected official.
References

Background
Under the Canada Health Act, prescription drugs administered in Canadian hospitals are provided at no cost to the patient. Outside of the hospital setting, most Canadians have some access to insurance coverage for prescription drugs through public and/or private drug plans.
The federal, provincial and territorial governments offer varying levels of coverage and decide who is covered and what the patient and plan pays. The publicly funded drug programs generally provide drug plan coverage for those most in need, based on age, income, and medical condition. Additionally, federal public drug plans provide coverage for veterans, First Nations and Inuit individuals, federally incarcerated offenders, the Royal Canadian Mounted Police, and the military.
Many Canadians and their family members have drug coverage linked to employment and private drug plans through an extended health benefit plan. Some Canadians may have no effective drug coverage and pay the full cost of prescription drugs.
| ACE’s National Survey on Arthritis Medications Reimbursement for People with Arthritis |
ACE conducted a 30-question online Survey between April 12, 2021 and April 30, 2021 of people with a physician- diagnosed arthritis in English and French. Respondents answered questions about the accessibility and affordability of reimbursement coverage for prescribed arthritis medications. Respondents required internet access to complete the Survey. Survey findings were analysed in aggregate (including incomplete Survey responses).
| Thank you to the people who took time to participate in this important community-led research and to our community partners who helped promote the Survey. |
Arthritis Consumer Experts received 319 responses, including 249 in English and 70 in French (69 were Quebec residents).
Respondents were patients living with different forms of arthritis, including osteoarthritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, lupus, juvenile idiopathic arthritis, fibromyalgia, Sjögren’s syndrome, scleroderma, and Behcet’s disease. The three most common forms of arthritis among respondents were rheumatoid arthritis (accounting for almost half of the respondents), osteoarthritis (1 in 10 or 14%), and ankylosing spondylitis (1 in 10 or 9%).
Forty-five per cent (5 in 10) of the respondents have been living with arthritis for over 10 years, while 14% (1 in 10) have been living with arthritis between 6-10 years and 41% (4 in 10) have been living with arthritis for 5 years or less.
In terms of gender, 8 in 10 (79%) of respondents were women, 2 in 10 (20%) were men, 0.33% respondents considered themselves as non-binary, 0.66% respondents selected other, and 0.66% respondents preferred not to answer this question.
A majority of respondents travel 2 times per year to see their rheumatologist (a little over 3 in 10 or 33%); followed by 3 in 10 or 30% who travel once a year to see their rheumatologist. One in 10 or 15% travel 3 times per year to see their rheumatologist, while 2 in 10 or 22% travel 4 or more times per year to see their rheumatologist.
Nearly 40% of respondents travel 1-10km to see their rheumatologist, followed by:
11-25km (28%)
26-50km (13%)
51-100km (8%)
101-250km (7%)
251-500km (3%)
500+km (1%)

Reimbursement coverage for arthritis medications
To identify the gaps in reimbursement coverage for arthritis medications in Canada, we asked Survey respondents to tell us how their arthritis prescription medications get reimbursed (paid for):
- nearly 4 in 10 or 39% respondents receive reimbursement through private drug plan insurance
- almost 3 in 10 or 27% respondents receive reimbursement through public drug plan insurance
- nearly 2 in 10 or 19% respondents receive reimbursement through both public drug plan and private drug plan insurance
- 1 in 10 or 12% respondents pay for medications out of their own pockets
- less than 1 in 10 or 3% respondents said someone else pays for their medications out of their own pocket
- English respondents were 2 times more likely to receive medications through both public drug plan and employer provided extended health benefits insurance (2 in 10 or 22% compared to 1 in 10 or 9%)
- Sixteen per cent (close to 2 in 10) of English respondents, compared to 2% (under 1 in 10) French respondents, pay for medications out of their own pockets
- French respondents were 2 times more likely to receive medications through public drug plan insurance (4 in 10 or 44% compared to 2 in 10 or 21%)


|
What advanced therapies are reimbursed by public and private drug plans? Advances in therapy for patients with inflammatory arthritis in the last 20 years have led to better disease management strategies and improved patient outcomes. Disease-modifying anti-rheumatic drugs (DMARDs) are a class of medications used to treat the signs and symptoms of rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis, and many other types of inflammatory arthritis. DMARDs now come in all “shapes and sizes” and can be taken by pill, self-injection, or infusion. These include synthetic DMARDs and biologic DMARDs. Synthetic DMARDs are divided into conventional synthetic DMARDS, which are small molecules and include traditional medications such as methotrexate, sulfasalazine, leflunomide, hydroxychloroquine, gold, and salts. Targeted synthetic DMARDs include only those medications that were specifically developed to target a particular molecular structure such as tofacitinib, baricitinib or apremilast, or agents not focused primarily on rheumatic diseases, such as imatinib or ibrutinib. What biologics (originators and biosimilars) are reimbursed by public and private drug plans? Biologic DMARDs are large proteins that target specific components of the immune response and are administered either by injection or infusion. Biologic DMARDs are classified into tumour necrosis factor (TNF) inhibitors and non-TNF inhibitors. The majority of biologic DMARDs currently approved for use in Canada are TNF inhibitor drugs and include adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab. Other available biologic DMARDs include the following non-TNF inhibitors: abatacept (T cell co-stimulatory inhibitor), anakinra (interleukin-1 receptor antagonist), rituximab (B lymphocyte-depleting drug), sarilumab, and tocilizumab (interleukin-6 receptor antagonists). Biologic DMARDS are now also divided into biologic originator DMARDs (boDMARDs) and biosimilar DMARDs (bsDMARDs). boDMARDS include abatacept, adalimumab, anakinra, certolizumab, etanercept, golimumab, infliximab, rituximab and tocilizumab. Several bsDMARDS are also available in Canada, including biosimilar versions of etanercept, infliximab, rituximab and adalimumab. Health Canada approved the biologics to be used in combination with one or more conventional synthetic DMARDs (usually methotrexate); all biologics except infliximab, golimumab, and rituximab are approved for use as monotherapy. Canadians spent $1.9 billion on 16.5 million claims for arthritis medications in 2017. The Conference Board of Canada recently published a report on access to arthritis medications: " Accessing Necessary Arthritis Medications: A Pan-Canadian Analysis."1 According to the report, of all arthritis medications, 22 per cent are paid for out-of-pocket, representing $248.9 million in uninsured spending. At the time of the report, there were 79 arthritis medications prescribed in Canada – private drug plans provide coverage for nearly all of these, while about 10 per cent of these medications are not accessible through public drug plans. Do respondents know what medications they take? The Survey found that 4 in 10 or 45% of respondents are taking a biologic (originator or biosimilar) and 1 in 10 or 11% of respondents are currently taking a targeted small molecule medicine. Out of the respondents who selected biologics as their current treatment therapy and knew what type it was, 7 in 10 or 73% respondents indicated they are on an originator biologic, while 3 in 10 or 27% indicated they are on a biosimilar biologic. Three in ten or 32% of applicable respondents “do not know” or “am not sure” what biologic medication(s) they are on. This is a red flag finding as it reveals a fundamental lack of patient understanding of their medication treatment. Ideally, when a patient and their healthcare specialist discuss whether to start, continue or stop taking a biologic therapy, the patient should be able to assess treatment (or no treatment) risk against benefit and have tools to enable them to discuss the pros and cons of the treatment with their healthcare team and the reimbursement access options. |
Two in 10 or 19% of respondents reported that the reimbursement access process was not easy and straightforward. While close to 7 in 10 or 68% of respondents strongly agreed or agreed that the process of getting reimbursement through public or private drug plans for their arthritis medication has generally been easy and straightforward.
More than 1 in 10 or 14% of respondents found it was neither easy nor tough.


What respondents told us:
“A map to guide patients through options for coverage (private, pharmacare, drug companies). Personally, my ‘coverage’ has changed numerous times depending on my own life stage – dependent, student, employee of small/medium/large businesses. Each life change, and each medication change, has come with a change in coverage.”
“The approval process for my medication can be somewhat confusing as several parties are involved - my rheumatologist, the provincial Medicare program, the provincial Seniors Pharmacare program, the drug company and of course the pharmacy. So it can be hard to keep track of who does what in the processes.”
“I think to have my medication for pain covered by Non-Insured Health Benefits for Indigenous (NIHB), I pay for my over-the-counter medication monthly. I have low income and not on social assistance.”
What were specific challenges with accessing reimbursement coverage for arthritis medication?
In the Survey, ACE asked respondents to share what specific difficulties they experienced when accessing coverage for their prescription arthritis medication. They could select multiple answers from a list of options. The options included:
- I have not experienced any difficulties: 138 (53%)
- Other (please specify): 57 (22%)
- My public or private drug plan declined to reimburse the cost of the arthritis medication that my rheumatologist said I needed: 36 (14%)
- I did not know who to talk to about reimbursement for my medications: 34 (13%)
- The entire process was difficult: 32 (12%)
- The forms I needed to fill out for reimbursement were confusing: 27 (10%)
- There were too many forms to fill out: 20 (6%)
- I was not given the right forms to fill out so could not apply for reimbursement: 8 (8%)

What respondents told us:
“I have to pay out of pocket until I meet my deductible for my private health insurance. The private health insurance pays until I meet my pharmacy requirements deductible. My husband retired last year and now my deductible has increased. With the biologic plus other medication I take, the monetary burden for my necessity medication has gone up significantly.”
“There was a great deal of stress because I knew I couldn't pay and yet I knew that without the drugs, I couldn't walk or function.”

Comparing different patient populations' experiences
A statistically significant higher number of respondents who identified as French reported that they did not experience any difficulties when it comes to getting coverage for their prescription arthritis medications. Specifically, 73% (7 in 10) of French respondents compared to nearly half or 47% (5 in 10) of English respondents reported not experiencing any difficulties.
There were also sharp differences with challenges in accessing reimbursement coverage for arthritis medication for black, Indigenous, and people of colour (BIPOC) respondents compared to the Survey findings for the general population.
Respondents who identified as BIPOC were 3 times more likely to report the forms they needed to fill out for reimbursement were confusing. Specifically, 29% (3 in 10) of BIPOC respondents reported that the forms they needed to fill out for reimbursement were confusing compared to 9% (1 in 10) white respondents. In addition, there were 4 times more BIPOC respondents who indicated there were too many forms to fill out with 24% of BIPOC respondents compared to 6% of white respondents. These findings underscore that the reimbursement forms may be too complicated to understand for people who do not speak English or French as a first language and the possibility of a lack of available translation services at various stages of the application process for medications reimbursement.

A deeper look at reimbursement access and affordability
ACE’s Survey findings showed a similar percentage of respondents on public and private drug plans who reported on how adequately their drug plan paid for arthritis medications and on their plan affordability.
Findings from respondents who have private drug plan coverage
Out of the 154 respondents who have private drug plan coverage, 70% (7 in 10) of respondents reported their private drug plan pays for most, or all, of their arthritis medications. While most respondents reported receiving adequate reimbursement coverage, 30% (3 in 10) of respondents reported their private drug plan did not adequately pay for their arthritis medications.
In terms of affordability, 54% (5 in 10) of respondents who identified as having private drug plan coverage reported their portion of private drug plan premium is affordable, while 19% (2 in 10) reported their portion as not affordable. Around 16% (2 in 10) of respondents who identified as having private drug plan coverage said their employer pays the entire premium of their private drug plan. 11% (1 in 10) indicated they do not know/am not sure if their insurance premium is affordable. It is important to note here that 48 respondents reported having both a public and a private drug plan coverage.
Findings from respondents who have public drug plan coverage
Out of the 136 respondents who have public drug plan coverage, 65% (over 6 in 10) reported that their public drug plan pays for most, or all, of their arthritis medications. While most respondents reported their public drug plan provided adequate reimbursement coverage, 35% (4 in 10) reported their public drug plan does not adequately pay for their arthritis medications. It is important to note here that 48 respondents reported having both a public and a private drug plan coverage.

The impact of poor reimbursement coverage for arthritis medications
In the Survey, ACE asked respondents what poor reimbursement coverage for prescribed arthritis medications forced them to do. They could select multiple answers from a list of options. The options included:
- None of the above. I have adequate reimbursement coverage for my prescribed arthritis medications: 158 (61%)
- Other: 44 (17%)
- Seek reimbursement coverage from pharmaceutical company: 33 (13%)
- Take less of the prescribed medication (for example skipping a dose or splitting pills): 21 (8%)
- Not fill your new prescription: 20 (8%)
- Start taking a different medication: 15 (5%)
- Not go on vacation: 15 (6%)
- Fill some prescription medications over others: 12 (5%)
- Borrow money to pay for medications: 12 (5%)
- Not renew your prescription: 9 (3%)
- Stop taking your prescribed medication: 7 (3%)
- Take medications prescribed for someone else: 2 (1%)

What respondents told us:
“Delayed purchasing a home due to cash flow being spent on meds rather than being saved.”
“I wait longer between treatments, so experience higher pain for a couple of months.”
“I have private coverage until I am 65, I don’t know what it will be like when I no longer have that private insurance in another 3 and a half years from now. I do worry about that, and if it will be affordable at that time or not. “
“We have just enough to make it each month. Some weeks are pretty tight on food to make sure I get my pills.”
Getting the information and support needed to navigate the arthritis medication reimbursement process
ACE asked respondents if they felt they had the information and support needed to understand reimbursement option and easily navigate the reimbursement process. Six in 10 or 61% of respondents reported they “strongly agree” or “agree” that they have the information and support needed.
By comparison, 17% of respondents said they “strongly disagree” or “disagree” that they have the information and support needed to understand reimbursement options and easily navigate the reimbursement process. According to respondents, here are some of the ways to help them get the information and support they need to better understand the reimbursement process:
- Making the websites for public or private drug plans more user friendly;
- Having clear information on all public and private programs and financial aides all in one place;
- Having information brochures with contact numbers and websites; and,
- Getting an explanation from healthcare providers.

What respondents told us:
“It is very confusing for young people who age out of the parents' coverage (I aged out of my mother's private coverage when I was no longer a full-time student) but don't have their own coverage yet. It will be even more confusing and worrisome when I turn 25 and am no longer covered by my dad's private coverage or OHIP+.”
“The extent of support I've received is the flyers that come with various biologic injections explaining whom to contact for manufacturers' support programs. The nurses who have provided these have, generally, not explaining any of the particulars, which vary by manufacturer. The ability to work with a specific pharmacist or nurse across medications (I'm sure I'm not the only arthritis patient who has tried many medications and often has to switch prescriptions) who has extensive and up-to-date training in seeking reimbursements would be very helpful. “
“I have no idea who when and how my new medication is going to get paid for. I have no idea if my old medication is good to be continued to pay for. One agency points the finger to the next. So I am left in the dark. Why can’t there be one agency to help me figure out my costs, help me with paperwork in a timely manner and send me regular (at least once a year) communication (either by phone, email or mail) regarding how my coverage is setup and if there’s any changes.”

Discussion
Addressing the challenges to reimbursement access
For government regulators and public and private drug plans to effectively review and approve new therapies and provide reimbursement access to treatment options, it is important for them to be aware of the unmet needs of arthritis patients; while at the same time, ensuring patients can afford the medicines they need.
Advanced therapies to treat inflammatory arthritis, such as biologics (originator and biosimilar) or targeted small molecule medications, deliver value both medically and socially. They also cost significantly more than other more conventional medicines. Over time, the introduction of new advanced therapies has resulted in increased public and private drug program spending on inflammatory arthritis treatment. For example, in 2018, biologic medicines accounted for less than 2% of prescribed drugs in Canada, yet the costs associated with them represented nearly 30% of Canada’s total prescription drug costs.2
Today, public drug plans are cost-managing formularies by restricting coverage for existing medications, delaying access to new medications and, at times, failing to address unmet treatment needs. Private insurers and pharmacy benefit managers have implemented a wide variety of cost containment programs and tools to manage plan sponsors’ drug plan costs, including case management and preferred pharmacy networks.
Survey findings show that reimbursement coverage accessibility and affordability for prescribed arthritis medications are causing hardship and forcing a significant percentage of respondents to make difficult choices to obtain and stay on their life-changing medications.
| Biologic medicines continue to be a growing budget pressure for public drug plans. As public and private drug plans continue to spend more and more of finite health dollars on biologics—and rightly so, given their efficacy and safety—it restricts their ability to provide coverage for existing medicines and to list any new medicines on their formulary. When ACE meets public and private drug plan managers, we highlight that biologics are life changing for patients with inflammatory arthritis and have vastly improved the treatment and prevention of disabling diseases like rheumatoid arthritis. We acknowledge that not only do biologics have higher prices, but they also are prescribed for longer durations to younger patients to manage chronic diseases. These higher and growing costs continue to put pressure on public and private drug plans. The use of biosimilars and the savings they generate are one solution to these challenges. The Patented Medicines Pricing Review Board has estimated that private and public drug plans across Canada could save from $332 million CDN to $1.81 billion CDN in the third year following biosimilar entry across a portfolio of product.3 The savings that can be generated through broader use of biosimilars can help public drug plans provide continued coverage to arthritis patients who need a biologic to manage their disease, allow them to cover new and innovative medicines, as well as expand access to existing medications. |

Advocating for improved reimbursement access
The findings from ACE’s National Survey on Arthritis Medications Reimbursement for People Living with Arthritis expose the need to amplify patient needs and priorities throughout the regulatory approval and reimbursement access process.
That requires patient organizations like ACE and individual patients to take action to support expanding access to arthritis medications. Patients can get involved by advocating for policies that move new medications through the most efficient review process, while still upholding strict safety and efficacy standards, and receive timely, equitable reimbursement coverage.
People with arthritis are the experts living with the disease: How do you get involved?
Patient input
ACE advocates for the Canadian patient community by responding to call for patient inputs. These inputs help inform policy decisions that affect the lives of Canadians living with arthritis. ACE has delivered more patient input submissions since its inception in 1999 than any other patient group organization in Canada.
Whenever public health authorities issue calls for patient input, ACE reaches out to the arthritis community for patient input of lived experience and submits these inputs to health authorities. To receive our calls for patient input submission, please subscribe to our JointHealth™ express newsletter.

How does your province or territory measure up?
Are there gaps in reimbursement coverage for medications approved for inflammatory arthritis such as rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis and juvenile idiopathic arthritis in your province or territory? ACE’s Arthritis Medications Report Card is designed to help Canadians measure their province or territories’ performance.
If your jurisdiction doesn’t measure up, ACE encourages you to write and speak to your elected provincial or federal representative about the lack of equitable reimbursement access and patient/physician choice in treating inflammatory forms of arthritis. Please go to ACE’s Advocacy page on our website where you can find information on how to contact your elected official.
|
For additional reading on the “therapy conversation” ACE’s JointHealth™ Education program was designed to engage, educate, and empower people living with arthritis to be an equal partner in the therapy conversation. We encourage you to select a course and join the growing list of graduates from JointHealth™ Education. |
| 1 | The Conference Board of Canada: Accessing Necessary Arthritis Medications – A Pan-Canadian Analysis: https://cms.ahpa.ca/tinymceuploads/source/COVID19/10608_Accessing_Necessary_Arthritis_Medications_A_Pan-Canadian_Analysis_2020.pdf |
| 2 | Patented Medicine Prices Review Board – Biologics in Canada https://www.canada.ca/content/dam/pmprb-cepmb/documents/reports-and-studies/chartbooks/biologics-part1-market-trends.pdf |
| 3 | Patented Medicines Prices Review Board. Potential Savings from Biosimilars in Canada https://www.pmprb-cepmb.gc.ca/CMFiles/NPDUIS/2017_Conference_Posters/post_6_biosim.pdf |
Listening to you
We hope you find this information of use. Please tell us what you think by writing to us or emailing us at feedback@jointhealth.org. Through your ongoing and active participation, ACE can make its work more relevant to all Canadians living with arthritis.
Update your email or postal address
Please let us know of any changes by contacting ACE at feedback@jointhealth.org. This will ensure that you continue to receive your free email or print copy of JointHealth™ insight.
Arthritis Consumer Experts (ACE)
Who We Are
Arthritis Consumer Experts (ACE) operates as a non-profit and provides free research based education and information to Canadians with arthritis. We help (em)power people living with all forms of arthritis to take control of their disease and to take action in healthcare and research decision making. ACE activities are guided by its members and led by people with arthritis, scientific and medical experts on the ACE Advisory Board. To learn more about ACE, visit www.jointhealth.org
Disclosures
Over the past 12 months, ACE received grants-in-aid from: Arthritis Research Canada, Amgen Canada, Canadian Biosimilars Forum, Canadian Institutes of Health Research, Canadian Rheumatology Association, Eli Lilly Canada, Fresenius Kabi Canada, Gilead Sciences Canada, Hoffman-La Roche Canada Ltd., Knowledge Translation Canada, Merck Canada, Novartis Canada, Pfizer Canada, Sandoz Canada, Sanofi Canada, St. Paul's Hospital (Vancouver), Teva Canada, UCB Canada, and the University of British Columbia.
ACE also received unsolicited donations from its community members (people with arthritis) across Canada.
ACE thanks funders for their support to help the nearly 6 million Canadians living with osteoarthritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and the many other forms of the disease.
Disclaimer
The material contained on this website is provided for general information only. This website should not be relied on to suggest a course of treatment for a particular individual or as a substitute for consultation with qualified health professionals who are familiar with your individual medical needs. Should you have any healthcare related questions, you should contact your physician. You should never disregard medical advice or delay in seeking it because of something you have read on this or any website.
This site may provide links to other Internet sites only for the convenience of World Wide Web users. ACE is not responsible for the availability or content of these external sites, nor does ACE endorse, warrant or guarantee the products, services or information described or offered at these other Internet sites.
Although the information presented on this website is believed to be accurate at the time it is posted, this website could include inaccuracies, typographical errors or out-of-date information. This website may be changed at any time without prior notice.

